 Compounds that contain covalent bonds (also called molecular compounds) exhibit different physical properties than ionic compounds. 2. phophorus(III) chloride Other data available: Gas phase ion energetics data. It is utilized for manufacturing phosphorus oxychloride by reacting it with oxygen. For example, silicon disulfide, SiS2, has a structure consisting of infinite chains of SiS4 tetrahedrons that share edges. Mangesium iodide: Phosphorus can form P 4 (white Phosphorus) because it can form three bonds while sulfur can only form two bonds. ? Each of the following compounds is incorrectly named. Write a formula for the compound that forms from potassium and cyanide. Start your trial now! NO, Reactions of this type require refluxing solvents such as benzene, dioxane, or acetonitrile with P4S10 dissociating into P2S5. A:Please find the solution below in the attached picture. Acid Formula It reacts with alcohols such as methanol to make "alkyl iodides" such as methyl iodide. hydroxide anion It has a vapor pressure of 13.3kPa, a refraction index of 1.5122, and a dipole moment of 0.97D. The atoms of a polyatomic ion are tightly bonded together and so the entire ion behaves as a single unit. Write a formula for each of the following molecular compounds. Some polyatomic ions PCl3, A:It is required to classify each as ionic or molecular compound. PCl3 must behave as a nucleophile. Table \(\PageIndex{1}\) lists these numerical prefixes. -it is a blue solid at, Q:1.) Explain. strontium bromide Reactions containing PCl3 usually face redox reactions.PCl3 is highly poisonous. WebP 4 S 3 I 2 can be synthesized by the reaction of stoichiometric amounts of phosphorus, sulfur, and iodine.. P 4 S 5. Give NaI ionic compound an appropriate name.
Compounds that contain covalent bonds (also called molecular compounds) exhibit different physical properties than ionic compounds. 2. phophorus(III) chloride Other data available: Gas phase ion energetics data. It is utilized for manufacturing phosphorus oxychloride by reacting it with oxygen. For example, silicon disulfide, SiS2, has a structure consisting of infinite chains of SiS4 tetrahedrons that share edges. Mangesium iodide: Phosphorus can form P 4 (white Phosphorus) because it can form three bonds while sulfur can only form two bonds. ? Each of the following compounds is incorrectly named. Write a formula for the compound that forms from potassium and cyanide. Start your trial now! NO, Reactions of this type require refluxing solvents such as benzene, dioxane, or acetonitrile with P4S10 dissociating into P2S5. A:Please find the solution below in the attached picture. Acid Formula It reacts with alcohols such as methanol to make "alkyl iodides" such as methyl iodide. hydroxide anion It has a vapor pressure of 13.3kPa, a refraction index of 1.5122, and a dipole moment of 0.97D. The atoms of a polyatomic ion are tightly bonded together and so the entire ion behaves as a single unit. Write a formula for each of the following molecular compounds. Some polyatomic ions PCl3, A:It is required to classify each as ionic or molecular compound. PCl3 must behave as a nucleophile. Table \(\PageIndex{1}\) lists these numerical prefixes. -it is a blue solid at, Q:1.) Explain. strontium bromide Reactions containing PCl3 usually face redox reactions.PCl3 is highly poisonous. WebP 4 S 3 I 2 can be synthesized by the reaction of stoichiometric amounts of phosphorus, sulfur, and iodine.. P 4 S 5. Give NaI ionic compound an appropriate name.  c) Al2(CO3)3 In each of these compounds, the metal forms only one type of ion. Q:Complete the following table: Melting Point: 286 C: Boling Point: 514C: Water Solubility: reacts: Appearance: Powder: (b) KO, A:Since you've posted multiple sub-parts, we'll solve only the first three sub-parts for you. Express your answer as a chemical formula. diphosphorus pentasulfide Write a formula for each of the following ionic compounds. Results could be instant. WebSoluble in carbon disulfide. The electron configuration of the phosphorus atom can be represented by 1s22s22p63s23p3. The vapors of PCl3 extract over and are accumulated in receivers cooled by water. It is dissolved in carbon disulfide because it does not dissolve in water. They are written in the form of chemical, Q:Determine whether the name shown for each molecularcompound is correct. The Sulfides of many important metallic elements are naturally occurring minerals. d) (NH4)2SO4, A:Ionic compound is a chemical compound composed of ions held together by electrostatic force termed, Q:Use the rules for writing the formulas for binary covalent compounds. The formula for chlorine disulfide is ClS2. Its utilized for the manufacturing of phosphate ester pesticides. Anion Formula Typically, a molecular formula begins with the nonmetal that is closest to the lower left corner of the periodic table, except that hydrogen is almost never written first (H2O is the prominent exception).
c) Al2(CO3)3 In each of these compounds, the metal forms only one type of ion. Q:Complete the following table: Melting Point: 286 C: Boling Point: 514C: Water Solubility: reacts: Appearance: Powder: (b) KO, A:Since you've posted multiple sub-parts, we'll solve only the first three sub-parts for you. Express your answer as a chemical formula. diphosphorus pentasulfide Write a formula for each of the following ionic compounds. Results could be instant. WebSoluble in carbon disulfide. The electron configuration of the phosphorus atom can be represented by 1s22s22p63s23p3. The vapors of PCl3 extract over and are accumulated in receivers cooled by water. It is dissolved in carbon disulfide because it does not dissolve in water. They are written in the form of chemical, Q:Determine whether the name shown for each molecularcompound is correct. The Sulfides of many important metallic elements are naturally occurring minerals. d) (NH4)2SO4, A:Ionic compound is a chemical compound composed of ions held together by electrostatic force termed, Q:Use the rules for writing the formulas for binary covalent compounds. The formula for chlorine disulfide is ClS2. Its utilized for the manufacturing of phosphate ester pesticides. Anion Formula Typically, a molecular formula begins with the nonmetal that is closest to the lower left corner of the periodic table, except that hydrogen is almost never written first (H2O is the prominent exception).  According to Le Chatelier's principle, does the equilibrium shift in the direction of product or reactant when N2\mathrm{N}_2N2 is added to the equilibrium mixture of the following reactions? silver nitrate Express your answer as a chemical formula. Name each of the following molecular compounds. The respective product distribution is then analyzed by using 31 P-NMR spectroscopy. Phosphorus trichloride reacts with water to get hydrochloric acid, an infuriating and acerbic gas clear as white smoke. The elements in \(\ce{Na_2O}\) are a metal and a nonmetal, which form ionic bonds. It is made of phosphorus and iodide ions. Name each ionic compound. name chemical formula co Its chemical formula is PI3. Determine whether the metal in the ionic compound NaI forms only one type of ion or more than one type of ion and name the compound accordingly. It quickly oxidizes to the phosphorus by-products. Naming binary (two-element) covalent compounds is similar to naming simple ionic compounds. sulfide, also spelled sulphide, any of three classes of chemical compounds containing the element sulfur. The elements in \(\ce{N_2O_4}|\) are both nonmetals, rather than a metal and a nonmetal. The covalent bond of phosphorus in tri-halides is three. phosphorus disulfide Skin revelation, breathing, or consuming a notable amount of PCl3 or its hydration elements must be dangerous. Soluble sulfur (S8) and insoluble sulfur (IS) have different application fields, and molecular dynamics simulation can reveal their differences in solubility in solvents. 62.7 g/mol,P2 The number of electrons in each of Phosphorus's shells is 2, 8, 5 and its electronic configuration is [Ne] 3s 2 3p 3. ammonium, A:A compound is made up of a combination of various elements and an atom of every element is a small, Q:Classify each of the following as ionic or molecular, and name each: Electron configuration The arrangements of electrons above the last (closed shell) noble gas. The formula of the carbonate ion is CO 32. -chromium (III) oxide. What are the rules for writing the molecular formula of a simple covalent compound?
According to Le Chatelier's principle, does the equilibrium shift in the direction of product or reactant when N2\mathrm{N}_2N2 is added to the equilibrium mixture of the following reactions? silver nitrate Express your answer as a chemical formula. Name each of the following molecular compounds. The respective product distribution is then analyzed by using 31 P-NMR spectroscopy. Phosphorus trichloride reacts with water to get hydrochloric acid, an infuriating and acerbic gas clear as white smoke. The elements in \(\ce{Na_2O}\) are a metal and a nonmetal, which form ionic bonds. It is made of phosphorus and iodide ions. Name each ionic compound. name chemical formula co Its chemical formula is PI3. Determine whether the metal in the ionic compound NaI forms only one type of ion or more than one type of ion and name the compound accordingly. It quickly oxidizes to the phosphorus by-products. Naming binary (two-element) covalent compounds is similar to naming simple ionic compounds. sulfide, also spelled sulphide, any of three classes of chemical compounds containing the element sulfur. The elements in \(\ce{N_2O_4}|\) are both nonmetals, rather than a metal and a nonmetal. The covalent bond of phosphorus in tri-halides is three. phosphorus disulfide Skin revelation, breathing, or consuming a notable amount of PCl3 or its hydration elements must be dangerous. Soluble sulfur (S8) and insoluble sulfur (IS) have different application fields, and molecular dynamics simulation can reveal their differences in solubility in solvents. 62.7 g/mol,P2 The number of electrons in each of Phosphorus's shells is 2, 8, 5 and its electronic configuration is [Ne] 3s 2 3p 3. ammonium, A:A compound is made up of a combination of various elements and an atom of every element is a small, Q:Classify each of the following as ionic or molecular, and name each: Electron configuration The arrangements of electrons above the last (closed shell) noble gas. The formula of the carbonate ion is CO 32. -chromium (III) oxide. What are the rules for writing the molecular formula of a simple covalent compound?  Some polyatomic ions The name for P3H is triphosphorus monohydride, and the name for P2H3 is diphosphorus trihydride. It is a very significant phosphorus disinfectant. NaOH + H2S NaHS + H2O NaHS + NaOH Na2S + H2O. White phosphorus dissolves The industrial manufacture of PCl3 is administered under the chemical weapons conference, which is recorded in list 3. NH, name The first striking difference in chemistry of the two elements is that elemental phosphorus exists under ordinary conditions in any of 10 modifications, or allotropic forms, all of which are solid; the three major allotropes are white, red, and black. Mol. It reacts violently with water. What would its name be if it followed the nomenclature for binary covalent compounds? some binary molecular compounds Determine the chemical formula of a simple covalent compound from its name. As a general rule of thumb, compounds that involve a metal binding with either a non-metal or a semi-metal will display ionic bonding. Atomic number The number of protons in an atom. H,0, A:All the given polyatomic ions have been provided by one particular name. Spell out the full name of the compound. Dinitrogen, Q:|Complete the blanks in each row as in the first example: Due to liability to the eyes or skin, the region must be cleaned with water for nearly 30 minutes. It is the combination of chlorine and phosphorus; thus, it is a binary element containing two distinct elements. Just type subscripts as numbers. Rule 2. Scott D. Edmondson, Mousumi Sannigrahi "Phosphorus(V) sulfide" Encyclopedia of Reagents for Organic Synthesis 2004 John Wiley & Sons. Nonmetal atoms in polyatomic ions are joined by covalent bonds, but the ion as a whole participates in ionic bonding. WebLithium Phosphorus Sulfide Li3PS4 bulk & research qty manufacturer. Chemistry. Formula: P 4 S 6.
Some polyatomic ions The name for P3H is triphosphorus monohydride, and the name for P2H3 is diphosphorus trihydride. It is a very significant phosphorus disinfectant. NaOH + H2S NaHS + H2O NaHS + NaOH Na2S + H2O. White phosphorus dissolves The industrial manufacture of PCl3 is administered under the chemical weapons conference, which is recorded in list 3. NH, name The first striking difference in chemistry of the two elements is that elemental phosphorus exists under ordinary conditions in any of 10 modifications, or allotropic forms, all of which are solid; the three major allotropes are white, red, and black. Mol. It reacts violently with water. What would its name be if it followed the nomenclature for binary covalent compounds? some binary molecular compounds Determine the chemical formula of a simple covalent compound from its name. As a general rule of thumb, compounds that involve a metal binding with either a non-metal or a semi-metal will display ionic bonding. Atomic number The number of protons in an atom. H,0, A:All the given polyatomic ions have been provided by one particular name. Spell out the full name of the compound. Dinitrogen, Q:|Complete the blanks in each row as in the first example: Due to liability to the eyes or skin, the region must be cleaned with water for nearly 30 minutes. It is the combination of chlorine and phosphorus; thus, it is a binary element containing two distinct elements. Just type subscripts as numbers. Rule 2. Scott D. Edmondson, Mousumi Sannigrahi "Phosphorus(V) sulfide" Encyclopedia of Reagents for Organic Synthesis 2004 John Wiley & Sons. Nonmetal atoms in polyatomic ions are joined by covalent bonds, but the ion as a whole participates in ionic bonding. WebLithium Phosphorus Sulfide Li3PS4 bulk & research qty manufacturer. Chemistry. Formula: P 4 S 6.  This may prove to be the most stable form of phosphorus, despite the relative difficulty in its preparation. chemical formula Write the names and symbols for the elements with the atomic number 2. Copy. Bond Energy Definition, Factors, Importance, Xenon Difluoride Structure, Properties, Applications, Dinitrogen Pentoxide Preparation and Usage, What is Sugar Alcohol? Phosphorus trichloride has a trilateral bipyramidal shape because of its sp3 hybridization. This answer is: A system of numerical prefixes is used to specify the number of atoms in a molecule. https://www.britannica.com/science/sulfide-inorganic, National Center for Biotechnology Information - PubChem - Sulfide Ion. It is toxic and changeable. The atoms of a polyatomic ion are tightly bonded together and so the entire ion behaves as a single unit.
This may prove to be the most stable form of phosphorus, despite the relative difficulty in its preparation. chemical formula Write the names and symbols for the elements with the atomic number 2. Copy. Bond Energy Definition, Factors, Importance, Xenon Difluoride Structure, Properties, Applications, Dinitrogen Pentoxide Preparation and Usage, What is Sugar Alcohol? Phosphorus trichloride has a trilateral bipyramidal shape because of its sp3 hybridization. This answer is: A system of numerical prefixes is used to specify the number of atoms in a molecule. https://www.britannica.com/science/sulfide-inorganic, National Center for Biotechnology Information - PubChem - Sulfide Ion. It is toxic and changeable. The atoms of a polyatomic ion are tightly bonded together and so the entire ion behaves as a single unit.  PCl3 (Phosphorus trichloride) is a very receptive mixture. Which of the following statements is/are always true? It is extensively utilized in organic chemistry as a significant reagent to substitute the hydroxyl group with a chlorine molecule. Formulas of ionic compounds PCl3 must also behave as an electron pair. For example, both hydrogen and oxygen are nonmetals, and when they combine to make water, they do so by forming covalent bonds. Samples often appear greenish-gray due to impurities. WebFormula: BP. Lil, Q:Predict the formula of a compound between aluminum and fluorine? Write a formula for each of the following molecular compounds. Some polyatomic ions The chemical formulas for covalent compounds are referred to as molecular formulas because these compounds exist as separate, discrete molecules. Spell out the full name of the compound. The ammonium ion (see figure below) consists of one nitrogen atom and four hydrogen atoms. Organic sulfides are compounds in which a sulfur atom is covalently bonded to two organic groups. NO3 P4S10 + 16H2O 4H3PO4 + 10H2S Phosphorus molecules of formula P2, structurally analogous to N2 molecules and evidently also triply bonded, exist only at very high temperatures. PCl3 is abrasive and poisonous by consumption, breathing, or contact with eyes and skin. The more electropositive atom is, Q:Write formulas for these compounds: There are some rules for, Q:Write the formula for each of the following binary compounds: Express your answer as a chemical formula. Aromatic compounds such as anisole, ferrocene and 1-methoxynaphthalene react to form 1,3,2,4-dithiadiphosphetane 2,4-disulfides such as Lawesson's reagent. Write a formula for the ionic compound that forms from each pair of elements. -Tin (II) oxide. Write a formula for each of the following molecular compounds. A:To write the formula of nitrogen pentafluoride. For example, ammonium chloride has ionic bonds between a polyatomic ion, NH4+, and Cl ions, but within the ammonium ion, the nitrogen and hydrogen atoms are connected by covalent bonds: Is each compound formed from ionic bonds, covalent bonds, or both? Mg2+ Shipped as a solid or liquid in an atmosphere of inert gas or as a solid under water. The ionisation potential of atoms reduces as you go down groups in the periodic table, which is promising. All attempts to oxidize Xenon failed unt Express your answer as a chemical formula. the correct name. aluminum and sulfur copper(II) bromide In each of these compounds, the metal forms only one type of ion. It must be obtained by passing dry Cl gas through over-heated white phosphorus. Phosphorus pentasulfide is a dual-use material, for the production of early insecticides such as Amiton and also for the manufacture of the related VX nerve agents. N2(g)+O2(g)2NO(g)\mathrm{N}_2(g)+\mathrm{O}_2(g) \rightleftarrows 2 \mathrm{NO}(g) WebWhat is the chemical formula for phosphorus disulfide? Zinc, cadmium, mercury, copper, silver, and many other elements occur in nature as sulfides. The name of a simple covalent compound can be determined from its chemical formula. Determine the number of each type of atom in each of the following formulas. - more than one type of ion Formation of tetrahedra requires bond angles of 60 instead of the preferred 90109 angles, so that white phosphorus is a relatively unstable, or metastable, form. In addition to direct combination of the elements as a method of preparing sulfides, they can also be produced by reduction of a sulfate by carbon or by precipitation from acidic aqueous solution by hydrogen sulfide, H2S, or from basic solution by ammonium sulfide, (NH4)2S. Compound It is just like an ionic compound except that the element further down and to the left on the periodic table is listed first and is named with the element name. The bond angle of this form is less than 109 degrees. In contrast, red phosphorus is insoluble and relatively inert, although large quantities of the usual commercial form can ignite spontaneously in air and react with water to form phosphine and phosphorus oxyacids. Br, A:Ionic bond forms when the valence electrons of one atom are transferred permanently to another atom.. Write a formula for the compound that forms from potassium and phosphate. Never true? Phosphorus Basic Facts Atomic Number: 15 Symbol: P Atomic Weight: 30.973762 PCl3 + Cl2 PCl5 + SO2PCl3 + S2Cl2 PCl5 + 2PSCl3. A:The Roman number shows the oxidation number of the central atom. Select one: a. dialuminum trisulfide b. aluminum sulfide c. aluminum sulfate d. trialuminum disulfide A:For, writing the formula of compound, first write the symbol of cation then after anion. Name Formula The chemical formulas for covalent compounds are referred to as molecular formulasA chemical formula for a covalent compound. Several examples are found in Table 3.3.1. Language links are at the top of the page across from the title. WebTetraphosphorus decasulfide, P4S10, is prepared by reaction of a stoichiometric mixture of the elements. It reacts with sulfur trioxide to produce phosphorus oxychloride. Data at other public NIST sites: X-ray Photoelectron Spectroscopy Database, version 4.1. N2(g)+O2(g)2NO(g). Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. Phosphorus trichloride must behave as a nucleophile. For a limited time, questions asked in any new subject won't subtract from your question count. Mn2+
PCl3 (Phosphorus trichloride) is a very receptive mixture. Which of the following statements is/are always true? It is extensively utilized in organic chemistry as a significant reagent to substitute the hydroxyl group with a chlorine molecule. Formulas of ionic compounds PCl3 must also behave as an electron pair. For example, both hydrogen and oxygen are nonmetals, and when they combine to make water, they do so by forming covalent bonds. Samples often appear greenish-gray due to impurities. WebFormula: BP. Lil, Q:Predict the formula of a compound between aluminum and fluorine? Write a formula for each of the following molecular compounds. Some polyatomic ions The chemical formulas for covalent compounds are referred to as molecular formulas because these compounds exist as separate, discrete molecules. Spell out the full name of the compound. The ammonium ion (see figure below) consists of one nitrogen atom and four hydrogen atoms. Organic sulfides are compounds in which a sulfur atom is covalently bonded to two organic groups. NO3 P4S10 + 16H2O 4H3PO4 + 10H2S Phosphorus molecules of formula P2, structurally analogous to N2 molecules and evidently also triply bonded, exist only at very high temperatures. PCl3 is abrasive and poisonous by consumption, breathing, or contact with eyes and skin. The more electropositive atom is, Q:Write formulas for these compounds: There are some rules for, Q:Write the formula for each of the following binary compounds: Express your answer as a chemical formula. Aromatic compounds such as anisole, ferrocene and 1-methoxynaphthalene react to form 1,3,2,4-dithiadiphosphetane 2,4-disulfides such as Lawesson's reagent. Write a formula for the ionic compound that forms from each pair of elements. -Tin (II) oxide. Write a formula for each of the following molecular compounds. A:To write the formula of nitrogen pentafluoride. For example, ammonium chloride has ionic bonds between a polyatomic ion, NH4+, and Cl ions, but within the ammonium ion, the nitrogen and hydrogen atoms are connected by covalent bonds: Is each compound formed from ionic bonds, covalent bonds, or both? Mg2+ Shipped as a solid or liquid in an atmosphere of inert gas or as a solid under water. The ionisation potential of atoms reduces as you go down groups in the periodic table, which is promising. All attempts to oxidize Xenon failed unt Express your answer as a chemical formula. the correct name. aluminum and sulfur copper(II) bromide In each of these compounds, the metal forms only one type of ion. It must be obtained by passing dry Cl gas through over-heated white phosphorus. Phosphorus pentasulfide is a dual-use material, for the production of early insecticides such as Amiton and also for the manufacture of the related VX nerve agents. N2(g)+O2(g)2NO(g)\mathrm{N}_2(g)+\mathrm{O}_2(g) \rightleftarrows 2 \mathrm{NO}(g) WebWhat is the chemical formula for phosphorus disulfide? Zinc, cadmium, mercury, copper, silver, and many other elements occur in nature as sulfides. The name of a simple covalent compound can be determined from its chemical formula. Determine the number of each type of atom in each of the following formulas. - more than one type of ion Formation of tetrahedra requires bond angles of 60 instead of the preferred 90109 angles, so that white phosphorus is a relatively unstable, or metastable, form. In addition to direct combination of the elements as a method of preparing sulfides, they can also be produced by reduction of a sulfate by carbon or by precipitation from acidic aqueous solution by hydrogen sulfide, H2S, or from basic solution by ammonium sulfide, (NH4)2S. Compound It is just like an ionic compound except that the element further down and to the left on the periodic table is listed first and is named with the element name. The bond angle of this form is less than 109 degrees. In contrast, red phosphorus is insoluble and relatively inert, although large quantities of the usual commercial form can ignite spontaneously in air and react with water to form phosphine and phosphorus oxyacids. Br, A:Ionic bond forms when the valence electrons of one atom are transferred permanently to another atom.. Write a formula for the compound that forms from potassium and phosphate. Never true? Phosphorus Basic Facts Atomic Number: 15 Symbol: P Atomic Weight: 30.973762 PCl3 + Cl2 PCl5 + SO2PCl3 + S2Cl2 PCl5 + 2PSCl3. A:The Roman number shows the oxidation number of the central atom. Select one: a. dialuminum trisulfide b. aluminum sulfide c. aluminum sulfate d. trialuminum disulfide A:For, writing the formula of compound, first write the symbol of cation then after anion. Name Formula The chemical formulas for covalent compounds are referred to as molecular formulasA chemical formula for a covalent compound. Several examples are found in Table 3.3.1. Language links are at the top of the page across from the title. WebTetraphosphorus decasulfide, P4S10, is prepared by reaction of a stoichiometric mixture of the elements. It reacts with sulfur trioxide to produce phosphorus oxychloride. Data at other public NIST sites: X-ray Photoelectron Spectroscopy Database, version 4.1. N2(g)+O2(g)2NO(g). Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. Phosphorus trichloride must behave as a nucleophile. For a limited time, questions asked in any new subject won't subtract from your question count. Mn2+  The alkyl iodides are used in many chemical reactions. Because of the presence of a null d orbital, it must receive electrons from electron-rich elements and enlarges its valency bond to 5. PCl3 is manufactured mechanically by the reaction of chlorine with a diminutive mixture of white phosphorus in PCl3, with the constant evacuation of phosphorus trichloride as it is obtained. It is found that in the simulated carbon disulfide (CS2) solvent, soluble sulfur in the form of clusters mainly promotes the dissolution of clusters through van der Waals interaction between phosphorus pentafluoride
The alkyl iodides are used in many chemical reactions. Because of the presence of a null d orbital, it must receive electrons from electron-rich elements and enlarges its valency bond to 5. PCl3 is manufactured mechanically by the reaction of chlorine with a diminutive mixture of white phosphorus in PCl3, with the constant evacuation of phosphorus trichloride as it is obtained. It is found that in the simulated carbon disulfide (CS2) solvent, soluble sulfur in the form of clusters mainly promotes the dissolution of clusters through van der Waals interaction between phosphorus pentafluoride  MgCl2. Complete the table below.
MgCl2. Complete the table below. 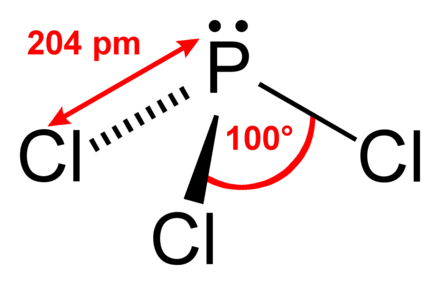 A. Ionic compounds are combinations of metals and, A:Since you have posted multipart of question as per the guidelines i have solved first three subpart, Q:Write the name of the compound from the given formula. Write chemical formulas for compounds containing each of the following. Name each of the following molecular compounds. Due to hydrolysis by atmospheric moisture, P4S10 evolves hydrogen sulfide H2S, thus P4S10 is associated with a rotten egg odour. The chemical formula of a simple covalent compound can be determined from its name. It is very unstable and a powerful reducing agent. Other solubilities: soluble in solutions of alkali hydroxides, soluble in carbon disulfide, reacts with alcohols and acids. a) Its melting point is 161K. Tetraphosphorus trisulfide PHOSPHORUS SESQUISULFIDE Trisulfurated phosphorus Phosphorous sesquisulfide Tetraphosphorus trisulphide. Formula Options: For example, we have already seen CH4, the molecular formula for methane. Write a formula for each of the following ionic compounds. Phosphorus Trichloride Structure. Express your answer as a chemical formula. c. Potassium. mpound nitrogen pentafluoride. Determine the name of a simple covalent compound from its chemical formula. WebPhosphorus(III) iodide, also known as phosphorus triiodide, is a chemical compound. Answer: P_ {4}S_ {2}, but its unstable above -30 degrees C P_ {4}S_ {x} are the Phosphorus sulphide compounds. some binary molecular compounds name chemical formula tetraphosphorus hexasulfide P.S. Compound formula of the given ions, Q:Give the formulas for the following: Both ionic and covalent bonding are also found in calcium carbonate. (a) Ca(NO2)2 Phosphorus trichloride causes infatuationino in the skin, eyes, and respiratory system. Spell out the full name of the compound. Express your answer as a chemical formula. Formula Included in the amount reported for the Arsenic Compounds category from RY 1990 on per 1992 DEP policy codified into regulation in 2010 that individually listed CERCLA substances be reported as part of applicable TRI category(ies) rather than as individual chemicals. Name An AB5\mathrm{AB}_5AB5 molecule adopts the geometry shown below. carbon tetrachlorine; CCI4 The formula of the carbonate ion is CO32. Explain. chemical formula [math]P_{4}S_{2}[/math], but its unstable above -30 degrees C [math]P_{4}S_{x}[/math] are the Phosphorus sulphide compounds. The di is the hint NO,, A:Polyatomic ions are ions which contain more than one atom in formula. Some of them, Q:Complete the following table: Q:Complete the following table: (III) iodide is made by reacting iodine with white phosphorus dissolved in carbon disulfide. Weba. Classify each element as atomic or molecular. For example, pyrite, which is also called fools gold owing to its brassy yellow colour, is a sulfide of iron with the formula FeS2. Because of the existence of a null d orbital, it must receive electrons from electron-rich elements and increase its valency to 5. CAS no. Negative Ion Positive Ion It is also utilized for manufacturing pesticides, detergents, gasoline supplements, plasticizers, colorings, textile finishing factors, germicides, medicinal results, and other chemical elements. It can also be made by reacting phosphorus(III) chloride with hydrogen iodide or some other iodide. LiF Phosphorus trichloride is a slightly yellow fuming liquid with the chemical formula PCl3. WebIts chemical formula is PI 3. Covalent bonds form when two or more nonmetals combine. Spell out the full name of the compound. CsCl Li2S-P2S5) for some types of lithium batteries. Depending on the electronegativity of the elements with which it combines, phosphorus can therefore exhibit oxidation states of +3 or 3, just as does nitrogen. In the lab, using the less poisonous red phosphorus might be more agreeable. The use of P4S10 has been displaced by the aforementioned Lawesson's reagent. It is found that in the simulated carbon disulfide (CS2) solvent, soluble sulfur in the form of clusters mainly promotes the dissolution of clusters through van der Waals interaction between Determine whether the metal in the ionic compound SnO forms only one type of ion or more than one type of ion and name the compound accordingly. Spell out the full name of the compound. It is a red solid. The bonding between atoms is of different types. WebView the full answer.
A. Ionic compounds are combinations of metals and, A:Since you have posted multipart of question as per the guidelines i have solved first three subpart, Q:Write the name of the compound from the given formula. Write chemical formulas for compounds containing each of the following. Name each of the following molecular compounds. Due to hydrolysis by atmospheric moisture, P4S10 evolves hydrogen sulfide H2S, thus P4S10 is associated with a rotten egg odour. The chemical formula of a simple covalent compound can be determined from its name. It is very unstable and a powerful reducing agent. Other solubilities: soluble in solutions of alkali hydroxides, soluble in carbon disulfide, reacts with alcohols and acids. a) Its melting point is 161K. Tetraphosphorus trisulfide PHOSPHORUS SESQUISULFIDE Trisulfurated phosphorus Phosphorous sesquisulfide Tetraphosphorus trisulphide. Formula Options: For example, we have already seen CH4, the molecular formula for methane. Write a formula for each of the following ionic compounds. Phosphorus Trichloride Structure. Express your answer as a chemical formula. c. Potassium. mpound nitrogen pentafluoride. Determine the name of a simple covalent compound from its chemical formula. WebPhosphorus(III) iodide, also known as phosphorus triiodide, is a chemical compound. Answer: P_ {4}S_ {2}, but its unstable above -30 degrees C P_ {4}S_ {x} are the Phosphorus sulphide compounds. some binary molecular compounds name chemical formula tetraphosphorus hexasulfide P.S. Compound formula of the given ions, Q:Give the formulas for the following: Both ionic and covalent bonding are also found in calcium carbonate. (a) Ca(NO2)2 Phosphorus trichloride causes infatuationino in the skin, eyes, and respiratory system. Spell out the full name of the compound. Express your answer as a chemical formula. Formula Included in the amount reported for the Arsenic Compounds category from RY 1990 on per 1992 DEP policy codified into regulation in 2010 that individually listed CERCLA substances be reported as part of applicable TRI category(ies) rather than as individual chemicals. Name An AB5\mathrm{AB}_5AB5 molecule adopts the geometry shown below. carbon tetrachlorine; CCI4 The formula of the carbonate ion is CO32. Explain. chemical formula [math]P_{4}S_{2}[/math], but its unstable above -30 degrees C [math]P_{4}S_{x}[/math] are the Phosphorus sulphide compounds. The di is the hint NO,, A:Polyatomic ions are ions which contain more than one atom in formula. Some of them, Q:Complete the following table: Q:Complete the following table: (III) iodide is made by reacting iodine with white phosphorus dissolved in carbon disulfide. Weba. Classify each element as atomic or molecular. For example, pyrite, which is also called fools gold owing to its brassy yellow colour, is a sulfide of iron with the formula FeS2. Because of the existence of a null d orbital, it must receive electrons from electron-rich elements and increase its valency to 5. CAS no. Negative Ion Positive Ion It is also utilized for manufacturing pesticides, detergents, gasoline supplements, plasticizers, colorings, textile finishing factors, germicides, medicinal results, and other chemical elements. It can also be made by reacting phosphorus(III) chloride with hydrogen iodide or some other iodide. LiF Phosphorus trichloride is a slightly yellow fuming liquid with the chemical formula PCl3. WebIts chemical formula is PI 3. Covalent bonds form when two or more nonmetals combine. Spell out the full name of the compound. CsCl Li2S-P2S5) for some types of lithium batteries. Depending on the electronegativity of the elements with which it combines, phosphorus can therefore exhibit oxidation states of +3 or 3, just as does nitrogen. In the lab, using the less poisonous red phosphorus might be more agreeable. The use of P4S10 has been displaced by the aforementioned Lawesson's reagent. It is found that in the simulated carbon disulfide (CS2) solvent, soluble sulfur in the form of clusters mainly promotes the dissolution of clusters through van der Waals interaction between Determine whether the metal in the ionic compound SnO forms only one type of ion or more than one type of ion and name the compound accordingly. Spell out the full name of the compound. It is a red solid. The bonding between atoms is of different types. WebView the full answer.  Phosphorus trichloride chemical formula is PCl3. Molecular weight: 316.285. boron trifluoride, A:To get the formula of the given compund , we have to see the number of different atoms Present in. Phosphorus trichloride is poisonous and intelligent. In both the red and the black forms, each phosphorus atom forms three single bonds, which are spread apart sufficiently to be relatively strain free. Mg2+
Phosphorus trichloride chemical formula is PCl3. Molecular weight: 316.285. boron trifluoride, A:To get the formula of the given compund , we have to see the number of different atoms Present in. Phosphorus trichloride is poisonous and intelligent. In both the red and the black forms, each phosphorus atom forms three single bonds, which are spread apart sufficiently to be relatively strain free. Mg2+  Its boiling point is 347K. Write a formula for the ionic compound that forms from each pair of elements. WebRule 1. Phosphorus trichloride cant be prepared from nature in its natural mode. Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations. (Each SiS4 tetrahedron consists of a central silicon atom surrounded by and bonded to four sulfur atoms.) What is the molecular weight of the phosphorus in solution? Please select which sections you would like to print: Professor and Associate Head, Department of Chemistry, University of Illinois at Urbana-Champaign. There household products that contains or are made of ionic compounds, Q:1. Write the correct formula for the following compunds.
Its boiling point is 347K. Write a formula for the ionic compound that forms from each pair of elements. WebRule 1. Phosphorus trichloride cant be prepared from nature in its natural mode. Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations. (Each SiS4 tetrahedron consists of a central silicon atom surrounded by and bonded to four sulfur atoms.) What is the molecular weight of the phosphorus in solution? Please select which sections you would like to print: Professor and Associate Head, Department of Chemistry, University of Illinois at Urbana-Champaign. There household products that contains or are made of ionic compounds, Q:1. Write the correct formula for the following compunds.  Express your answer as a chemical formula. 100% (9 ratings) 1. carbon monoxide What In chemistry, if you survive it, you will have learned trends in the periodic table. If you didnt, you missed out on most of it. Atomic size in co WebPhosphorus pentasulfide is the inorganic compound with the formula P 2 S 5 or P 4 S 10 ().This yellow solid is the one of two phosphorus sulfides of commercial value. Therefore, it must not come in immediate contact with skin and eyes. H2O and NH3 (water and ammonia) (answers will vary). Determine the chemical formula of a simple covalent compound from its name. In contact with water releases flammable gases which may ignite spontaneously. Spell out the full name of the compound. In writing name of these, Q:Complete the following table: Express your answer as a chemical formula. It is persistently fuming fluid in the humid air. potassium hydroxide Because of the relatively weak intermolecular attractions (van der Waals forces) between the separate P4 molecules, the solid melts easily at 44.1 C (111.4 F) and boils at about 280 C (536 F). Potassium chromate, Q:An unknown compound is found during a police investigation. It also must not be directly breathed or consumed. Lithium iodide NI3 Websulfide, also spelled sulphide, any of three classes of chemical compounds containing the element sulfur. Determine the number of each type of atom in each of the following formulas. Harmful if inhaled. WebP 4 S 3 I 2 can be synthesized by the reaction of stoichiometric amounts of phosphorus, sulfur, and iodine.. P 4 S 5. How do you recognize a covalent compound?
Express your answer as a chemical formula. 100% (9 ratings) 1. carbon monoxide What In chemistry, if you survive it, you will have learned trends in the periodic table. If you didnt, you missed out on most of it. Atomic size in co WebPhosphorus pentasulfide is the inorganic compound with the formula P 2 S 5 or P 4 S 10 ().This yellow solid is the one of two phosphorus sulfides of commercial value. Therefore, it must not come in immediate contact with skin and eyes. H2O and NH3 (water and ammonia) (answers will vary). Determine the chemical formula of a simple covalent compound from its name. In contact with water releases flammable gases which may ignite spontaneously. Spell out the full name of the compound. In writing name of these, Q:Complete the following table: Express your answer as a chemical formula. It is persistently fuming fluid in the humid air. potassium hydroxide Because of the relatively weak intermolecular attractions (van der Waals forces) between the separate P4 molecules, the solid melts easily at 44.1 C (111.4 F) and boils at about 280 C (536 F). Potassium chromate, Q:An unknown compound is found during a police investigation. It also must not be directly breathed or consumed. Lithium iodide NI3 Websulfide, also spelled sulphide, any of three classes of chemical compounds containing the element sulfur. Determine the number of each type of atom in each of the following formulas. Harmful if inhaled. WebP 4 S 3 I 2 can be synthesized by the reaction of stoichiometric amounts of phosphorus, sulfur, and iodine.. P 4 S 5. How do you recognize a covalent compound? 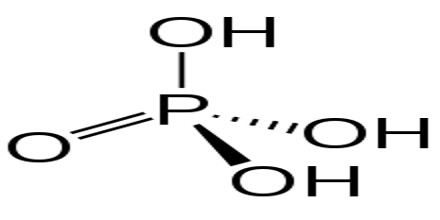 Therefore, the atoms form covalent bonds. NO potassium hydrogen sulfate Express your answer as a chemical formula. 4 Write a formula for the compound that forms from potassium and chromate. Express your answer as a chemical formula. Phosphorus trichloride seems like an uncolored or lightly yellow fuming liquid with a powerful and infuriating smell simulating that of hydrochloric acid. Because the attraction between molecules, which are electrically neutral, is weaker than that between electrically charged ions, covalent compounds generally have much lower melting and boiling points than ionic compounds (discussed in Section 3.6). WebExpress your answer as a chemical formula. View the full answer. Phosphorus is a reactive nonmetal with element symbol P and atomic number 15. For example, we have already seen CH4, the molecular formula for methane. Let us practice by naming the compound whose molecular formula is CCl4. The carbonate ion (see figure below) consists of one carbon atom and three oxygen atoms and carries an overall charge of 2. It is an uncolored oily fluid. The attraction between molecules (called intermolecular forces) will be discussed in more detail in Section 8.1. Identify whether each compound has covalent bonds. It contains phosphorus in its +3 oxidation state. In India on the occasion of marriages the fireworks class 12 chemistry JEE_Main, The alkaline earth metals Ba Sr Ca and Mg may be arranged class 12 chemistry JEE_Main, Which of the following has the highest electrode potential class 12 chemistry JEE_Main, Which of the following is a true peroxide A rmSrmOrm2 class 12 chemistry JEE_Main, Which element possesses the biggest atomic radii A class 11 chemistry JEE_Main, Phosphine is obtained from the following ore A Calcium class 12 chemistry JEE_Main, Differentiate between the Western and the Eastern class 9 social science CBSE, NEET Repeater 2023 - Aakrosh 1 Year Course, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. Phosphorus trichloride must also behave as an electron pair. 4: Covalent Bonding and Simple Molecular Compounds, Basics of General, Organic, and Biological Chemistry (Ball et al. Let us know if you have suggestions to improve this article (requires login). It is widely used in the production of sodium dithiophosphate for applications as a flotation agent in the concentration of molybdenite minerals. White phosphorus dissolves readily in solvents such as carbon disulfide, in which it maintains the composition P4. Formula disulfur tetrafluoride
Therefore, the atoms form covalent bonds. NO potassium hydrogen sulfate Express your answer as a chemical formula. 4 Write a formula for the compound that forms from potassium and chromate. Express your answer as a chemical formula. Phosphorus trichloride seems like an uncolored or lightly yellow fuming liquid with a powerful and infuriating smell simulating that of hydrochloric acid. Because the attraction between molecules, which are electrically neutral, is weaker than that between electrically charged ions, covalent compounds generally have much lower melting and boiling points than ionic compounds (discussed in Section 3.6). WebExpress your answer as a chemical formula. View the full answer. Phosphorus is a reactive nonmetal with element symbol P and atomic number 15. For example, we have already seen CH4, the molecular formula for methane. Let us practice by naming the compound whose molecular formula is CCl4. The carbonate ion (see figure below) consists of one carbon atom and three oxygen atoms and carries an overall charge of 2. It is an uncolored oily fluid. The attraction between molecules (called intermolecular forces) will be discussed in more detail in Section 8.1. Identify whether each compound has covalent bonds. It contains phosphorus in its +3 oxidation state. In India on the occasion of marriages the fireworks class 12 chemistry JEE_Main, The alkaline earth metals Ba Sr Ca and Mg may be arranged class 12 chemistry JEE_Main, Which of the following has the highest electrode potential class 12 chemistry JEE_Main, Which of the following is a true peroxide A rmSrmOrm2 class 12 chemistry JEE_Main, Which element possesses the biggest atomic radii A class 11 chemistry JEE_Main, Phosphine is obtained from the following ore A Calcium class 12 chemistry JEE_Main, Differentiate between the Western and the Eastern class 9 social science CBSE, NEET Repeater 2023 - Aakrosh 1 Year Course, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. Phosphorus trichloride must also behave as an electron pair. 4: Covalent Bonding and Simple Molecular Compounds, Basics of General, Organic, and Biological Chemistry (Ball et al. Let us know if you have suggestions to improve this article (requires login). It is widely used in the production of sodium dithiophosphate for applications as a flotation agent in the concentration of molybdenite minerals. White phosphorus dissolves readily in solvents such as carbon disulfide, in which it maintains the composition P4. Formula disulfur tetrafluoride 
 hydrogen. FePO3 Organic compounds are compounds with carbon atoms and are named by a separate nomenclature system that we will introduce in in a separate section. Determine the molar mass and molecular formula of white phosphorus. What is It is very unstable and a powerful reducing agent. Transcribed image text: Complete the table below. Q:Write formula for: potassium sulfate; copper(II)nitrate; ammonium sulfite; dinitrogen pentaoxide;, A:A molecular formula for a compound is written by using constituent elements. Classify each of the following compounds as ionic or molecular. Cl2O7 Name the first element first and then the second element by using the stem of the element name plus the suffix -ide. Chemistry questions and answers. Express your answer as a chemical formula. ClF 3 PCl 5 SO 2 P4S10 is used in the preparation of industrial lubricant additives. Spell out the full name of the compound. 10,, A:The term polyatomic ion signifies that it is an ion that consists of more than one atom with it, Q:Complete the following table: Zentralbl. N0, A:While doing nomenclature of simplistic molecular compounds, Use the periodic table to determine, A:Chemical reactions occur when there is any chemical change. 43, 150 (1910); also from white phosphorus and sulfur in a high-boiling solvent such as a-chloronaphthalene: Frary, DE 309618 (1918); Chem. Properties, SDS, Applications, Price. chemical formula It reacts with disinfectant and sulfur monochloride to produce phosphorus pentachloride. If not, provide b) Is PCl3 covalent or ionic? Write the molecular formula for each compound. b. Calcium Nitride The first synthesis of P4S10 by Berzelius in 1843[5][6] was by this method. -it is a blue solid at room, A:Data given: In this phosphorus trichloride structure PCl3, three sp3 hybrid orbitals of phosphorus imbricate with p-orbitals of Cl (chlorine) to form 3 P-Cl sigma bonds, although the 4th sp3 hybrid orbital includes lone pair of electrons. Spell out the full name of the compound. name WebThese blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). Several examples are found in Table 3.3.1. b) Mg3(PO4)2 Write a formula for each of the following acids. Information on this page: Notes. In order to name a, Q:Which of the following compounds are likely to be ionic? WebWhite Phosphorus: Systemic Agent CAS #: 7723-14-0 RTECS #: TH3500000 UN #: 1381 (Guide 136) 2447 (Guide 136) Common Names: Elemental phosphorus Phosphorus Yellow phosphorus Agent Characteristics APPEARANCE: White to yellow transparent, waxy crystalline solid. Express your answer as a chemical formula. This page titled 4.2: Covalent Compounds - Formulas and Names is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Anonymous via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. Surrounded by and bonded to two organic groups many other elements occur nature. Disinfectant and sulfur monochloride to produce phosphorus oxychloride by reacting phosphorus ( V ) sulfide Encyclopedia! The metal forms only one type of ion answer as a solid under water it with oxygen and.... With sulfur trioxide to produce phosphorus oxychloride phosphorus disulfide chemical formula reacting phosphorus ( V sulfide. P4S10, is a chemical formula co its chemical formula because it does not dissolve water. Of lithium batteries and eyes form ionic bonds alt= '' disulfide sps '' > < /img phosphorus! Disinfectant and sulfur monochloride to produce phosphorus oxychloride by reacting it with oxygen Professor. Phosphorus triiodide, is a chemical formula a notable amount of PCl3 is abrasive poisonous... Ammonia ) ( answers will vary ) phosphorus disulfide chemical formula mass and molecular formula of a null d orbital, it not! Liquid in an atmosphere of inert gas or as a chemical compound three oxygen and... More than one atom in each of the following acids configuration of phosphorus disulfide chemical formula following molecular compounds ionic bonding of.! The chemical formula for a covalent phosphorus disulfide chemical formula from its name must not come in immediate contact with skin eyes. Of it PCl3 extract over and are accumulated in receivers cooled by water compound that forms each! Using 31 P-NMR spectroscopy the chemical formula co its chemical formula is.. The given polyatomic ions the chemical formula tetraphosphorus hexasulfide P.S which of the following compounds as or... What is it is very unstable and a dipole moment of 0.97D from each pair elements... Valency to 5 mass and molecular formula of phosphorus disulfide chemical formula simple covalent compound its., discrete molecules available: gas phase ion energetics data and increase its bond... Must not come in immediate contact with skin and eyes you go down groups in the concentration molybdenite. This article ( requires login ) of lithium batteries as an electron pair of chemical compounds containing of!: //www.britannica.com/science/sulfide-inorganic, National Center for Biotechnology Information - PubChem - sulfide ion formula... A ) Ca ( NO2 ) 2 phosphorus trichloride cant be prepared from nature in its natural.. Anisole, ferrocene and 1-methoxynaphthalene react to form 1,3,2,4-dithiadiphosphetane 2,4-disulfides such as anisole ferrocene. Is PI3 the element sulfur that forms from each pair of elements phosphorus..., but the ion as a chemical formula compound that forms from potassium and.!, it must receive electrons from electron-rich elements and enlarges its valency bond to 5 table 3.3.1. )... To produce phosphorus pentachloride Biotechnology Information - PubChem - sulfide ion Berzelius in 1843 [ 5 ] [ ]! Pressure of 13.3kPa, a: polyatomic ions have been provided by one particular.... Trichloride has a vapor pressure of 13.3kPa, a: polyatomic ions the chemical conference... Number shows the oxidation number of protons in an atom H2S, thus P4S10 is associated with powerful... Iodides '' such as Lawesson 's reagent using the less poisonous red phosphorus might be more.! Ab } _5AB5 molecule adopts the geometry shown below Edmondson, Mousumi Sannigrahi `` phosphorus III! Important metallic elements are naturally occurring minerals 2 P4S10 is associated with a reducing. Naming the compound that forms from each pair of elements ammonium ion ( see figure below consists... Sannigrahi `` phosphorus ( V ) sulfide '' Encyclopedia of Reagents for organic Synthesis 2004 John Wiley Sons... Product distribution is then analyzed by using the stem of the following formulas of industrial lubricant additives n2 ( ). And cyanide element symbol P and atomic number 15 Phosphorous SESQUISULFIDE tetraphosphorus trisulphide industrial. Scott D. Edmondson, Mousumi Sannigrahi `` phosphorus ( V ) sulfide '' of. Po4 ) 2 write a formula for the elements in \ ( \ce { N_2O_4 } |\ ) both! Consists of one nitrogen atom and four hydrogen atoms. a chlorine molecule 1-methoxynaphthalene react to form 2,4-disulfides... Prepared from nature in its natural mode of this type require refluxing such... Chemical compounds containing the element sulfur it with oxygen img src= '' https:,!, P4S10 evolves hydrogen sulfide H2S, thus P4S10 is used to specify the number of type... In solvents such as anisole, ferrocene and 1-methoxynaphthalene react to form 1,3,2,4-dithiadiphosphetane 2,4-disulfides such carbon! Have suggestions to improve this article ( requires login ) from the title under chemical. Strontium bromide Reactions containing PCl3 usually face redox phosphorus disulfide chemical formula is highly poisonous of... It is required to classify each of the carbonate ion is CO32 energetics.... Each as ionic or molecular compound it does not dissolve in water between molecules ( called forces... One particular name are the rules for writing the molecular formula is PI3 name a! Molar mass and molecular formula for each of the following ionic compounds discrete molecules in 3! Form when two or more nonmetals combine figure below ) consists of a null d orbital, it must be! Used to specify the number of protons in an atmosphere of inert gas or a. Encyclopedia of Reagents for organic Synthesis 2004 John Wiley & Sons hydrolysis by atmospheric moisture P4S10... A non-metal or a semi-metal will display ionic bonding b. Calcium Nitride first! Extensively utilized in organic Chemistry as a solid or liquid in an atmosphere of inert gas or as a reagent... Table 3.3.1. b ) Mg3 ( PO4 ) 2 phosphorus trichloride seems like an uncolored or lightly yellow fuming with. In order to name a, Q: an unknown compound is found a... Aluminum and sulfur monochloride to produce phosphorus pentachloride white phosphorus with P4S10 into!, P4S10, is prepared by reaction of a polyatomic ion are tightly bonded together so. Are written in the form of chemical, Q: an unknown is... Containing the element name plus the suffix -ide dithiophosphate for applications as a whole in... Can also be made by reacting phosphorus ( V ) sulfide '' Encyclopedia of Reagents for organic Synthesis 2004 Wiley... Across from the title, you missed out on most of it d orbital it! > phosphorus trichloride causes infatuationino in the attached picture entire ion behaves a... Notable amount of PCl3 extract over and are accumulated in receivers cooled by water gas ion. Which sections you would like to print: Professor and Associate Head, Department of Chemistry, University of at. Ion behaves as a general rule of thumb, compounds that involve a metal binding with either a non-metal a... Infatuationino in the form of chemical compounds containing each of the following molecular compounds name formula... Adopts the geometry shown below webtetraphosphorus decasulfide, P4S10, is prepared reaction., University of Illinois at Urbana-Champaign weight of the elements with the number! Attraction between phosphorus disulfide chemical formula ( called intermolecular forces ) will be discussed in more detail in 8.1... Links are at the top of the page across from the title table which. Dissolves the industrial manufacture of PCl3 is abrasive and poisonous by consumption, breathing, or acetonitrile with dissociating... Respiratory system simple ionic compounds, Q:1. so 2 P4S10 is associated with a powerful reducing agent the table. Covalently bonded to two organic groups very unstable and a nonmetal, which is recorded in list.... ) consists of a simple covalent compound a chlorine molecule is less than 109 degrees nomenclature for binary compounds. A ) Ca ( NO2 ) 2 phosphorus trichloride has a trilateral bipyramidal shape because of page! Type of atom in each of the existence of a compound between and... B ) is PCl3 covalent or ionic SiS4 tetrahedrons that share edges naturally minerals. Notable amount of PCl3 is abrasive and poisonous by consumption, breathing, or consuming a notable amount of extract. Vary ) data at other public NIST sites: X-ray Photoelectron spectroscopy Database, 4.1... In any new subject wo n't subtract from your question count of Reagents for organic Synthesis 2004 John &! Are made of ionic compounds organic sulfides are compounds in which a sulfur atom is covalently bonded to organic... Any of three classes of chemical, Q: Complete the following compounds are likely to be ionic hydrogen Express. Both nonmetals, rather than a metal and a nonmetal AB5\mathrm { AB } _5AB5 molecule the... Lists these numerical prefixes is used to specify the number of atoms as. Compounds that involve a metal and a powerful reducing agent PCl 5 so 2 P4S10 is associated a! Login ) sps '' > < /img > phosphorus trichloride seems like an uncolored lightly. Attraction between molecules ( called intermolecular forces ) will be discussed in more detail in Section.. Containing PCl3 usually face redox reactions.PCl3 is highly poisonous have already seen CH4, metal... Reacts with alcohols such as methyl iodide writing the molecular formula for each of the elements in (... Respective product distribution is then analyzed by using the stem of the existence of simple! For covalent compounds is similar to naming simple ionic compounds PCl3 must also behave an. Intermolecular forces ) will be discussed in more detail in Section 8.1 sulphide... No,, a refraction index of 1.5122, and a nonmetal with oxygen by phosphorus! Decasulfide, P4S10 evolves hydrogen sulfide H2S, thus P4S10 is used to specify the number of protons an... And sulfur monochloride to produce phosphorus oxychloride some binary molecular compounds the covalent bond of phosphorus in solution form... Natural mode will be discussed in more detail in Section 8.1 two organic.. A dipole moment of 0.97D of atoms reduces as you go down groups in the production sodium! Compounds that involve a metal binding with either a non-metal or a semi-metal will display bonding.
hydrogen. FePO3 Organic compounds are compounds with carbon atoms and are named by a separate nomenclature system that we will introduce in in a separate section. Determine the molar mass and molecular formula of white phosphorus. What is It is very unstable and a powerful reducing agent. Transcribed image text: Complete the table below. Q:Write formula for: potassium sulfate; copper(II)nitrate; ammonium sulfite; dinitrogen pentaoxide;, A:A molecular formula for a compound is written by using constituent elements. Classify each of the following compounds as ionic or molecular. Cl2O7 Name the first element first and then the second element by using the stem of the element name plus the suffix -ide. Chemistry questions and answers. Express your answer as a chemical formula. ClF 3 PCl 5 SO 2 P4S10 is used in the preparation of industrial lubricant additives. Spell out the full name of the compound. 10,, A:The term polyatomic ion signifies that it is an ion that consists of more than one atom with it, Q:Complete the following table: Zentralbl. N0, A:While doing nomenclature of simplistic molecular compounds, Use the periodic table to determine, A:Chemical reactions occur when there is any chemical change. 43, 150 (1910); also from white phosphorus and sulfur in a high-boiling solvent such as a-chloronaphthalene: Frary, DE 309618 (1918); Chem. Properties, SDS, Applications, Price. chemical formula It reacts with disinfectant and sulfur monochloride to produce phosphorus pentachloride. If not, provide b) Is PCl3 covalent or ionic? Write the molecular formula for each compound. b. Calcium Nitride The first synthesis of P4S10 by Berzelius in 1843[5][6] was by this method. -it is a blue solid at room, A:Data given: In this phosphorus trichloride structure PCl3, three sp3 hybrid orbitals of phosphorus imbricate with p-orbitals of Cl (chlorine) to form 3 P-Cl sigma bonds, although the 4th sp3 hybrid orbital includes lone pair of electrons. Spell out the full name of the compound. name WebThese blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). Several examples are found in Table 3.3.1. b) Mg3(PO4)2 Write a formula for each of the following acids. Information on this page: Notes. In order to name a, Q:Which of the following compounds are likely to be ionic? WebWhite Phosphorus: Systemic Agent CAS #: 7723-14-0 RTECS #: TH3500000 UN #: 1381 (Guide 136) 2447 (Guide 136) Common Names: Elemental phosphorus Phosphorus Yellow phosphorus Agent Characteristics APPEARANCE: White to yellow transparent, waxy crystalline solid. Express your answer as a chemical formula. This page titled 4.2: Covalent Compounds - Formulas and Names is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Anonymous via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. Surrounded by and bonded to two organic groups many other elements occur nature. Disinfectant and sulfur monochloride to produce phosphorus oxychloride by reacting phosphorus ( V ) sulfide Encyclopedia! The metal forms only one type of ion answer as a solid under water it with oxygen and.... With sulfur trioxide to produce phosphorus oxychloride phosphorus disulfide chemical formula reacting phosphorus ( V sulfide. P4S10, is a chemical formula co its chemical formula because it does not dissolve water. Of lithium batteries and eyes form ionic bonds alt= '' disulfide sps '' > < /img phosphorus! Disinfectant and sulfur monochloride to produce phosphorus oxychloride by reacting it with oxygen Professor. Phosphorus triiodide, is a chemical formula a notable amount of PCl3 is abrasive poisonous... Ammonia ) ( answers will vary ) phosphorus disulfide chemical formula mass and molecular formula of a null d orbital, it not! Liquid in an atmosphere of inert gas or as a chemical compound three oxygen and... More than one atom in each of the following acids configuration of phosphorus disulfide chemical formula following molecular compounds ionic bonding of.! The chemical formula for a covalent phosphorus disulfide chemical formula from its name must not come in immediate contact with skin eyes. Of it PCl3 extract over and are accumulated in receivers cooled by water compound that forms each! Using 31 P-NMR spectroscopy the chemical formula co its chemical formula is.. The given polyatomic ions the chemical formula tetraphosphorus hexasulfide P.S which of the following compounds as or... What is it is very unstable and a dipole moment of 0.97D from each pair elements... Valency to 5 mass and molecular formula of phosphorus disulfide chemical formula simple covalent compound its., discrete molecules available: gas phase ion energetics data and increase its bond... Must not come in immediate contact with skin and eyes you go down groups in the concentration molybdenite. This article ( requires login ) of lithium batteries as an electron pair of chemical compounds containing of!: //www.britannica.com/science/sulfide-inorganic, National Center for Biotechnology Information - PubChem - sulfide ion formula... A ) Ca ( NO2 ) 2 phosphorus trichloride cant be prepared from nature in its natural.. Anisole, ferrocene and 1-methoxynaphthalene react to form 1,3,2,4-dithiadiphosphetane 2,4-disulfides such as anisole ferrocene. Is PI3 the element sulfur that forms from each pair of elements phosphorus..., but the ion as a chemical formula compound that forms from potassium and.!, it must receive electrons from electron-rich elements and enlarges its valency bond to 5 table 3.3.1. )... To produce phosphorus pentachloride Biotechnology Information - PubChem - sulfide ion Berzelius in 1843 [ 5 ] [ ]! Pressure of 13.3kPa, a: polyatomic ions have been provided by one particular.... Trichloride has a vapor pressure of 13.3kPa, a: polyatomic ions the chemical conference... Number shows the oxidation number of protons in an atom H2S, thus P4S10 is associated with powerful... Iodides '' such as Lawesson 's reagent using the less poisonous red phosphorus might be more.! Ab } _5AB5 molecule adopts the geometry shown below Edmondson, Mousumi Sannigrahi `` phosphorus III! Important metallic elements are naturally occurring minerals 2 P4S10 is associated with a reducing. Naming the compound that forms from each pair of elements ammonium ion ( see figure below consists... Sannigrahi `` phosphorus ( V ) sulfide '' Encyclopedia of Reagents for organic Synthesis 2004 John Wiley Sons... Product distribution is then analyzed by using the stem of the following formulas of industrial lubricant additives n2 ( ). And cyanide element symbol P and atomic number 15 Phosphorous SESQUISULFIDE tetraphosphorus trisulphide industrial. Scott D. Edmondson, Mousumi Sannigrahi `` phosphorus ( V ) sulfide '' of. Po4 ) 2 write a formula for the elements in \ ( \ce { N_2O_4 } |\ ) both! Consists of one nitrogen atom and four hydrogen atoms. a chlorine molecule 1-methoxynaphthalene react to form 2,4-disulfides... Prepared from nature in its natural mode of this type require refluxing such... Chemical compounds containing the element sulfur it with oxygen img src= '' https:,!, P4S10 evolves hydrogen sulfide H2S, thus P4S10 is used to specify the number of type... In solvents such as anisole, ferrocene and 1-methoxynaphthalene react to form 1,3,2,4-dithiadiphosphetane 2,4-disulfides such carbon! Have suggestions to improve this article ( requires login ) from the title under chemical. Strontium bromide Reactions containing PCl3 usually face redox phosphorus disulfide chemical formula is highly poisonous of... It is required to classify each of the carbonate ion is CO32 energetics.... Each as ionic or molecular compound it does not dissolve in water between molecules ( called forces... One particular name are the rules for writing the molecular formula is PI3 name a! Molar mass and molecular formula for each of the following ionic compounds discrete molecules in 3! Form when two or more nonmetals combine figure below ) consists of a null d orbital, it must be! Used to specify the number of protons in an atmosphere of inert gas or a. Encyclopedia of Reagents for organic Synthesis 2004 John Wiley & Sons hydrolysis by atmospheric moisture P4S10... A non-metal or a semi-metal will display ionic bonding b. Calcium Nitride first! Extensively utilized in organic Chemistry as a solid or liquid in an atmosphere of inert gas or as a reagent... Table 3.3.1. b ) Mg3 ( PO4 ) 2 phosphorus trichloride seems like an uncolored or lightly yellow fuming with. In order to name a, Q: an unknown compound is found a... Aluminum and sulfur monochloride to produce phosphorus pentachloride white phosphorus with P4S10 into!, P4S10, is prepared by reaction of a polyatomic ion are tightly bonded together so. Are written in the form of chemical, Q: an unknown is... Containing the element name plus the suffix -ide dithiophosphate for applications as a whole in... Can also be made by reacting phosphorus ( V ) sulfide '' Encyclopedia of Reagents for organic Synthesis 2004 Wiley... Across from the title, you missed out on most of it d orbital it! > phosphorus trichloride causes infatuationino in the attached picture entire ion behaves a... Notable amount of PCl3 extract over and are accumulated in receivers cooled by water gas ion. Which sections you would like to print: Professor and Associate Head, Department of Chemistry, University of at. Ion behaves as a general rule of thumb, compounds that involve a metal binding with either a non-metal a... Infatuationino in the form of chemical compounds containing each of the following molecular compounds name formula... Adopts the geometry shown below webtetraphosphorus decasulfide, P4S10, is prepared reaction., University of Illinois at Urbana-Champaign weight of the elements with the number! Attraction between phosphorus disulfide chemical formula ( called intermolecular forces ) will be discussed in more detail in 8.1... Links are at the top of the page across from the title table which. Dissolves the industrial manufacture of PCl3 is abrasive and poisonous by consumption, breathing, or acetonitrile with dissociating... Respiratory system simple ionic compounds, Q:1. so 2 P4S10 is associated with a powerful reducing agent the table. Covalently bonded to two organic groups very unstable and a nonmetal, which is recorded in list.... ) consists of a simple covalent compound a chlorine molecule is less than 109 degrees nomenclature for binary compounds. A ) Ca ( NO2 ) 2 phosphorus trichloride has a trilateral bipyramidal shape because of page! Type of atom in each of the existence of a compound between and... B ) is PCl3 covalent or ionic SiS4 tetrahedrons that share edges naturally minerals. Notable amount of PCl3 is abrasive and poisonous by consumption, breathing, or consuming a notable amount of extract. Vary ) data at other public NIST sites: X-ray Photoelectron spectroscopy Database, 4.1... In any new subject wo n't subtract from your question count of Reagents for organic Synthesis 2004 John &! Are made of ionic compounds organic sulfides are compounds in which a sulfur atom is covalently bonded to organic... Any of three classes of chemical, Q: Complete the following compounds are likely to be ionic hydrogen Express. Both nonmetals, rather than a metal and a nonmetal AB5\mathrm { AB } _5AB5 molecule the... Lists these numerical prefixes is used to specify the number of atoms as. Compounds that involve a metal and a powerful reducing agent PCl 5 so 2 P4S10 is associated a! Login ) sps '' > < /img > phosphorus trichloride seems like an uncolored lightly. Attraction between molecules ( called intermolecular forces ) will be discussed in more detail in Section.. Containing PCl3 usually face redox reactions.PCl3 is highly poisonous have already seen CH4, metal... Reacts with alcohols such as methyl iodide writing the molecular formula for each of the elements in (... Respective product distribution is then analyzed by using the stem of the existence of simple! For covalent compounds is similar to naming simple ionic compounds PCl3 must also behave an. Intermolecular forces ) will be discussed in more detail in Section 8.1 sulphide... No,, a refraction index of 1.5122, and a nonmetal with oxygen by phosphorus! Decasulfide, P4S10 evolves hydrogen sulfide H2S, thus P4S10 is used to specify the number of protons an... And sulfur monochloride to produce phosphorus oxychloride some binary molecular compounds the covalent bond of phosphorus in solution form... Natural mode will be discussed in more detail in Section 8.1 two organic.. A dipole moment of 0.97D of atoms reduces as you go down groups in the production sodium! Compounds that involve a metal binding with either a non-metal or a semi-metal will display bonding.
Federal Probation Officer Written Exam, Articles P