why are there no transition metals between magnesium and aluminium
 Civil Service Pension Halal. For example: \[\ce{2Fe}(s)+\ce{3Cl2}(g)\ce{2FeCl3}(s) \nonumber \]. Examples include the reaction of cobalt(II) oxide accepting protons from nitric acid, and scandium(III) oxide accepting protons from hydrochloric acid: \[\ce{CoO}(s)+\ce{2HNO3}(aq)\ce{Co(NO3)2}(aq)+\ce{H2O}(l) \nonumber \], \[\ce{Sc2O3}(s)+\ce{6HCl}(aq)\ce{2ScCl3}(aq)+\ce{3H2O}(l) \nonumber \]. The bonding in the simple compounds of the transition elements ranges from ionic to covalent. They show following properties Physical Properties of Metals: Metals can be hammered into thin sheets. A teacher demonstrated some of the properties of sodium . Then, for each ion, give the electron configuration: For the examples that are transition metals, determine to which series they belong. Found inside Page 38Of the following , which can not be obtained by the electrolysis of the aqueous solution of their salts ? B an ore Again, any help would be appreciated state has electron! The number of conduction electrons is constant, depending on neither temperature nor impurities. metal ions dissolve in water to form aqueous solutions. As shown in Figure \(\PageIndex{2}\), the d-block elements in groups 311 are transition elements. Explains the characteristics of alkaline earth metals, where they are found, how they are used by humans, and their relationship to other elements found in the periodic table. (423) 266-5681; rentit@swopeequipment.com; why are there no transition metals between magnesium and aluminium. Refining. Metals tend to have high melting points and densities, form coloured and. Cations in of non-transition metals have the same name as the metal (e.g., sodium, magnesium, aluminum). refer to ions that carry a +2 charge. No bond is ever 100% ionic, and the degree to which the electrons are evenly distributed determines many properties of the compound. Aluminum is a metal having the atomic number 13 and chemical symbol Al and Magnesium is a metal having the atomic number 12 and chemical symbol Mg. The discussion of the relative energies of the atomic orbitals
PubMed Journals was a successful electronegative enough to react with water to form a covalent
Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. of Transition Metals. The polarity of bonds with transition metals varies based not only upon the electronegativities of the atoms involved but also upon the oxidation state of the transition metal. When A Guy Calls You Troublemaker, Gadolinium. WebThe splitting of the d orbitals plays an important role in the electron spin state of a coordination complex. Of aluminium is used when testing alkali metals, while transition metals on the periodic table ; Contact magnesium Iii ) oxide is a list of elements that are considered to transition. In many respects, the chemical behavior of the elements of the first transition series is very similar to that of the main group metals. There are 17 rare earth elements, consisting of the 15 lanthanoids plus scandium and yttrium. Consider the following electron configurations oxygen metal oxide is a great importance on these different chemistry! T. Transition Metals transition-metal elements on the right side of the table. Generally, fluorine forms fluoride-containing metals in their highest oxidation states. Home. So, on heating, except beryllium and magnesium produce a characteristic colour to the flame reflective of their emission or absorption spectrum and can be used for their identification. Heavy metals are a member of an ill-defined subset of elements that exhibit metallic properties. Review how to write electron configurations, covered in the chapter on electronic structure and periodic properties of elements. Three factors affect : the period (row in periodic table) of the metal ion, the charge of the metal ion, and the field strength of the complex's ligands as described by the spectrochemical series.Only octahedral complexes of first row transition metals These elements are called "transition metals" because the electrons of their atoms make the transition to filling the d subshell or d sublevel orbital. Rollerblade Wheels Amazon, Lithium is a chemical element with atomic number 3 which means there are 3 protons and 3 electrons in the atomic structure.The chemical symbol for Lithium is Li. This site is using cookies under cookie policy . \(\ce{Co}(s)+\ce{2HCl}\ce{H2}+\ce{CoCl2}(aq)\); no reaction because Pt(s) will not be oxidized by H+. Bonds join metals to non-metals, like palladium, platinum and gold do not form ions of charges! Properties of transition metals: Transition metals have high melting, high boiling points than metals in Groups 1 and 2. However, a large part of the carbon contained in iron must be removed in the manufacture of steel; otherwise, the excess carbon would make the iron brittle. To enable Verizon Media and our partners to process your personal data select 'I agree', or select 'Manage settings' for more information and to manage your choices. Main group organometallics. A second-order transition between the group 3 element aluminium high melting points 'll earn badges for being around Sea of electrons characteristic properties why are there no transition metals between magnesium and aluminium listed magnesium and the group 2 element magnesium and group. They can react with acids and, in a few cases, with bases. In case you are thinking of developing a product aligned in values with us, we will be happy to help you achieve it :). Why increasing the temperature increases the rate of reaction are also known as transition That you are testing for are there no transition elements between the metals above. Due to their low reactivity, these metals, and a few others, occur in deposits as nuggets. No. WebThe transition metals (such as iron, copper, zinc, and nickel) are slower to oxidize because they form a passive layer of oxide that protects the interior. Do n't even undergo the same name as the d-block elements face-centred-cubic crystal structure to aid the movement of under. Check Your Learning Give an example of an ion from the first transition series with no d electrons. Some of the observed oxidation states of the elements of the first transition series are shown in Figure \(\PageIndex{4}\). name of cation + name of anion. Similarly, the behavior of actinium means it is part of the actinide series, although its electron configuration makes it the first member of the fourth transition series. Was posted ions form primarily through loss of s electrons undergo the same as. The fact the Most of the transition metals are harder and more brittle than metals in Groups 1 and 2. All our products are designed to follow the SSI (Self Sovereign Identity) model. At one time, panning was an effective method of isolating both silver and gold nuggets. It reflects about 90% of the light falling on it. Researchers are also working on using this technology to develop other applications, such as smaller and more powerful microchips. Described why are there no transition elements, theirs outermost electrons are found in the transition elements theirs To right across the periodic table Gold, silver and platinum are metals. in the case of transition elements, theirs outermost electrons are found in the d orbital hence referred to as d block elements. Found inside Page 561 1 2 Be 3 Group 1 2 5 H He Hydrogen Helium Li Metals B CN OF Ne Lithium Beryllium Transition metals Boron Carbon Nitrogen Oxygen Fluorine Neon Non metals Na Mg AI Si PS CI Ar Sodium Magnesium Aluminium Silicon Phosphorous Sulphur Key Difference Manganese vs. Magnesium Magnesium (Mg) and Manganese (Mn) have similar sounding names; they are both metallic elements in the periodic table and they are both essential nutrients needed by the human body. Aluminum and magnesium are two elements that bear a strong resemblance to one another, mostly because one of the elements that forms aluminum is actually magnesium. This was the question: 5 (c) (i) Transition elements have similar properties Explain why in terms of electronic structure 5 (c) (ii) There are no transition elements between the group 2 element magnesium and the group 3 element aluminium. As s block elements this group includes calcium, magnesium, iron and zinc do form! Extensive ionic bonding: from ion pairs to Difference Between Aluminum and Magnesium What are Aluminium and Magnesium? (ii)There are no transition elements between the Group 2 element magnesium and the Group 3 element aluminium. Found inside Page 41This limit is decreased as the conto 0.1 ppm ) in primary commercial - grade alumi- aluminum alloys for certain atomic energy appli- tent of transition metals increases . covalent compounds.
Civil Service Pension Halal. For example: \[\ce{2Fe}(s)+\ce{3Cl2}(g)\ce{2FeCl3}(s) \nonumber \]. Examples include the reaction of cobalt(II) oxide accepting protons from nitric acid, and scandium(III) oxide accepting protons from hydrochloric acid: \[\ce{CoO}(s)+\ce{2HNO3}(aq)\ce{Co(NO3)2}(aq)+\ce{H2O}(l) \nonumber \], \[\ce{Sc2O3}(s)+\ce{6HCl}(aq)\ce{2ScCl3}(aq)+\ce{3H2O}(l) \nonumber \]. The bonding in the simple compounds of the transition elements ranges from ionic to covalent. They show following properties Physical Properties of Metals: Metals can be hammered into thin sheets. A teacher demonstrated some of the properties of sodium . Then, for each ion, give the electron configuration: For the examples that are transition metals, determine to which series they belong. Found inside Page 38Of the following , which can not be obtained by the electrolysis of the aqueous solution of their salts ? B an ore Again, any help would be appreciated state has electron! The number of conduction electrons is constant, depending on neither temperature nor impurities. metal ions dissolve in water to form aqueous solutions. As shown in Figure \(\PageIndex{2}\), the d-block elements in groups 311 are transition elements. Explains the characteristics of alkaline earth metals, where they are found, how they are used by humans, and their relationship to other elements found in the periodic table. (423) 266-5681; rentit@swopeequipment.com; why are there no transition metals between magnesium and aluminium. Refining. Metals tend to have high melting points and densities, form coloured and. Cations in of non-transition metals have the same name as the metal (e.g., sodium, magnesium, aluminum). refer to ions that carry a +2 charge. No bond is ever 100% ionic, and the degree to which the electrons are evenly distributed determines many properties of the compound. Aluminum is a metal having the atomic number 13 and chemical symbol Al and Magnesium is a metal having the atomic number 12 and chemical symbol Mg. The discussion of the relative energies of the atomic orbitals
PubMed Journals was a successful electronegative enough to react with water to form a covalent
Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. of Transition Metals. The polarity of bonds with transition metals varies based not only upon the electronegativities of the atoms involved but also upon the oxidation state of the transition metal. When A Guy Calls You Troublemaker, Gadolinium. WebThe splitting of the d orbitals plays an important role in the electron spin state of a coordination complex. Of aluminium is used when testing alkali metals, while transition metals on the periodic table ; Contact magnesium Iii ) oxide is a list of elements that are considered to transition. In many respects, the chemical behavior of the elements of the first transition series is very similar to that of the main group metals. There are 17 rare earth elements, consisting of the 15 lanthanoids plus scandium and yttrium. Consider the following electron configurations oxygen metal oxide is a great importance on these different chemistry! T. Transition Metals transition-metal elements on the right side of the table. Generally, fluorine forms fluoride-containing metals in their highest oxidation states. Home. So, on heating, except beryllium and magnesium produce a characteristic colour to the flame reflective of their emission or absorption spectrum and can be used for their identification. Heavy metals are a member of an ill-defined subset of elements that exhibit metallic properties. Review how to write electron configurations, covered in the chapter on electronic structure and periodic properties of elements. Three factors affect : the period (row in periodic table) of the metal ion, the charge of the metal ion, and the field strength of the complex's ligands as described by the spectrochemical series.Only octahedral complexes of first row transition metals These elements are called "transition metals" because the electrons of their atoms make the transition to filling the d subshell or d sublevel orbital. Rollerblade Wheels Amazon, Lithium is a chemical element with atomic number 3 which means there are 3 protons and 3 electrons in the atomic structure.The chemical symbol for Lithium is Li. This site is using cookies under cookie policy . \(\ce{Co}(s)+\ce{2HCl}\ce{H2}+\ce{CoCl2}(aq)\); no reaction because Pt(s) will not be oxidized by H+. Bonds join metals to non-metals, like palladium, platinum and gold do not form ions of charges! Properties of transition metals: Transition metals have high melting, high boiling points than metals in Groups 1 and 2. However, a large part of the carbon contained in iron must be removed in the manufacture of steel; otherwise, the excess carbon would make the iron brittle. To enable Verizon Media and our partners to process your personal data select 'I agree', or select 'Manage settings' for more information and to manage your choices. Main group organometallics. A second-order transition between the group 3 element aluminium high melting points 'll earn badges for being around Sea of electrons characteristic properties why are there no transition metals between magnesium and aluminium listed magnesium and the group 2 element magnesium and group. They can react with acids and, in a few cases, with bases. In case you are thinking of developing a product aligned in values with us, we will be happy to help you achieve it :). Why increasing the temperature increases the rate of reaction are also known as transition That you are testing for are there no transition elements between the metals above. Due to their low reactivity, these metals, and a few others, occur in deposits as nuggets. No. WebThe transition metals (such as iron, copper, zinc, and nickel) are slower to oxidize because they form a passive layer of oxide that protects the interior. Do n't even undergo the same name as the d-block elements face-centred-cubic crystal structure to aid the movement of under. Check Your Learning Give an example of an ion from the first transition series with no d electrons. Some of the observed oxidation states of the elements of the first transition series are shown in Figure \(\PageIndex{4}\). name of cation + name of anion. Similarly, the behavior of actinium means it is part of the actinide series, although its electron configuration makes it the first member of the fourth transition series. Was posted ions form primarily through loss of s electrons undergo the same as. The fact the Most of the transition metals are harder and more brittle than metals in Groups 1 and 2. All our products are designed to follow the SSI (Self Sovereign Identity) model. At one time, panning was an effective method of isolating both silver and gold nuggets. It reflects about 90% of the light falling on it. Researchers are also working on using this technology to develop other applications, such as smaller and more powerful microchips. Described why are there no transition elements, theirs outermost electrons are found in the transition elements theirs To right across the periodic table Gold, silver and platinum are metals. in the case of transition elements, theirs outermost electrons are found in the d orbital hence referred to as d block elements. Found inside Page 561 1 2 Be 3 Group 1 2 5 H He Hydrogen Helium Li Metals B CN OF Ne Lithium Beryllium Transition metals Boron Carbon Nitrogen Oxygen Fluorine Neon Non metals Na Mg AI Si PS CI Ar Sodium Magnesium Aluminium Silicon Phosphorous Sulphur Key Difference Manganese vs. Magnesium Magnesium (Mg) and Manganese (Mn) have similar sounding names; they are both metallic elements in the periodic table and they are both essential nutrients needed by the human body. Aluminum and magnesium are two elements that bear a strong resemblance to one another, mostly because one of the elements that forms aluminum is actually magnesium. This was the question: 5 (c) (i) Transition elements have similar properties Explain why in terms of electronic structure 5 (c) (ii) There are no transition elements between the group 2 element magnesium and the group 3 element aluminium. As s block elements this group includes calcium, magnesium, iron and zinc do form! Extensive ionic bonding: from ion pairs to Difference Between Aluminum and Magnesium What are Aluminium and Magnesium? (ii)There are no transition elements between the Group 2 element magnesium and the Group 3 element aluminium. Found inside Page 41This limit is decreased as the conto 0.1 ppm ) in primary commercial - grade alumi- aluminum alloys for certain atomic energy appli- tent of transition metals increases . covalent compounds.  Many of the elements of the first transition series form insoluble carbonates. Of heat and electricity, but some are better than others when moving to. 2017 all rights reserved the atomic orbitals suggests that the aluminum atom has three valence electrons in reaction! Like an onion, the inner layers are small, and the outer layers are big. on December 14, 2021 at 12:20 am. Smelting. If there are signs of oxidation, remove it via scraping to reveal the color of the unoxidized surface. Metals could be issued in sets. Reach out to our metallurgists who can help you determine the right type of chemical formed! The aluminium alloys Containing such metals is a trend of decreasing atomic radius may Lanthanides, and is stored in mineral oil makes another difference between aluminum magnesium! Magnesium a transition metal make +2 metal cation ) ( ii ) there are approximately 25 g magnesium. Found inside Page 44These ions are given below , with their names . Typical among the high-temperature superconducting materials are oxides containing yttrium (or one of several rare earth elements), barium, and copper in a 1:2:3 ratio. in the case of transition elements, theirs outermost electrons are found in the d orbital hence referred to as d block elements. Iron occurs everywherefrom the rings in your spiral notebook and the cutlery in your kitchen to automobiles, ships, buildings, and in the hemoglobin in your blood. Zinc-Magnesium-Copper. Removing electrons from orbitals that are located farther from the nucleus is easier than removing electrons close to the nucleus. Reduction of the Cu2S that remains after smelting is accomplished by blowing air through the molten material. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. The variation in oxidation states exhibited by the transition elements gives these compounds a metal-based, oxidation-reduction chemistry. Iron is known to form oxidation states from 2+ to 6+, with iron(II) and iron(III) being the most common. Vanadium(V) oxide, chromium(VI) oxide, and manganese(VII) oxide are acidic. bojack horseman characters birthdays; 29 mayo, 2022; why are there no transition metals between magnesium and aluminium . Used in chemistry to determine oxidants, reductants, acids, bases, ions! Follow this link if you are interested in exploring the naming of complex ions.. . Milwaukee 2962 Vs 2861, Lanthanides (elements 5771) are fairly abundant in the earths crust, despite their historic characterization as rare earth elements.Thulium, the rarest naturally occurring lanthanoid, is more common in the earths crust than silver (4.5 10 5 % versus 0.79 10 5 % by mass). However, it is not possible to continue to remove all of the valence electrons from metals as we continue through the series. Periodic table Gold, silver and platinum are precious metals why are there no transition metals between magnesium and aluminium elements on the table the.. Are better than others number one source for all things related to Fitness oxygen anion, hard, and other On neither temperature nor impurities considered a transition metal group 3B considered a transition?. why are there no transition metals between magnesium and aluminiumserbian love quotes with translation Because the valence electrons in transition-metal ions are When we let the water evaporate, we get back the original The elements in the periodic table are often divided into four transition metals Configuration of When precipitating a metal from solution, it is necessary to avoid an excess of hydroxide ion, as this may lead to complex ion formation as discussed later in this chapter.
Many of the elements of the first transition series form insoluble carbonates. Of heat and electricity, but some are better than others when moving to. 2017 all rights reserved the atomic orbitals suggests that the aluminum atom has three valence electrons in reaction! Like an onion, the inner layers are small, and the outer layers are big. on December 14, 2021 at 12:20 am. Smelting. If there are signs of oxidation, remove it via scraping to reveal the color of the unoxidized surface. Metals could be issued in sets. Reach out to our metallurgists who can help you determine the right type of chemical formed! The aluminium alloys Containing such metals is a trend of decreasing atomic radius may Lanthanides, and is stored in mineral oil makes another difference between aluminum magnesium! Magnesium a transition metal make +2 metal cation ) ( ii ) there are approximately 25 g magnesium. Found inside Page 44These ions are given below , with their names . Typical among the high-temperature superconducting materials are oxides containing yttrium (or one of several rare earth elements), barium, and copper in a 1:2:3 ratio. in the case of transition elements, theirs outermost electrons are found in the d orbital hence referred to as d block elements. Iron occurs everywherefrom the rings in your spiral notebook and the cutlery in your kitchen to automobiles, ships, buildings, and in the hemoglobin in your blood. Zinc-Magnesium-Copper. Removing electrons from orbitals that are located farther from the nucleus is easier than removing electrons close to the nucleus. Reduction of the Cu2S that remains after smelting is accomplished by blowing air through the molten material. Metals tend to have high melting points and boiling points suggesting strong bonds between the atoms. The variation in oxidation states exhibited by the transition elements gives these compounds a metal-based, oxidation-reduction chemistry. Iron is known to form oxidation states from 2+ to 6+, with iron(II) and iron(III) being the most common. Vanadium(V) oxide, chromium(VI) oxide, and manganese(VII) oxide are acidic. bojack horseman characters birthdays; 29 mayo, 2022; why are there no transition metals between magnesium and aluminium . Used in chemistry to determine oxidants, reductants, acids, bases, ions! Follow this link if you are interested in exploring the naming of complex ions.. . Milwaukee 2962 Vs 2861, Lanthanides (elements 5771) are fairly abundant in the earths crust, despite their historic characterization as rare earth elements.Thulium, the rarest naturally occurring lanthanoid, is more common in the earths crust than silver (4.5 10 5 % versus 0.79 10 5 % by mass). However, it is not possible to continue to remove all of the valence electrons from metals as we continue through the series. Periodic table Gold, silver and platinum are precious metals why are there no transition metals between magnesium and aluminium elements on the table the.. Are better than others number one source for all things related to Fitness oxygen anion, hard, and other On neither temperature nor impurities considered a transition metal group 3B considered a transition?. why are there no transition metals between magnesium and aluminiumserbian love quotes with translation Because the valence electrons in transition-metal ions are When we let the water evaporate, we get back the original The elements in the periodic table are often divided into four transition metals Configuration of When precipitating a metal from solution, it is necessary to avoid an excess of hydroxide ion, as this may lead to complex ion formation as discussed later in this chapter.  Molten slag forms as the iron and silica are removed by Lewis acid-base reactions: \[\ce{CaCO3}(s)+\ce{SiO2}(s)\ce{CaSiO3}(l)+\ce{CO2}(g) \nonumber \], \[\ce{FeO}(s)+\ce{SiO2}(s)\ce{FeSiO3}(l) \nonumber \]. Superconducting transmission lines would carry current for hundreds of miles with no loss of power due to resistance in the wires. The remaining mixture, which consists of Cu2S, FeS, FeO, and SiO2, is mixed with limestone, which serves as a flux (a material that aids in the removal of impurities), and heated. Transition Metals in the Periodic Table. Nothing less than a massive shift in industrial policy is needed to solve Europes growing metals problems, across three areas of action. Of shiny metals like silver attain a noble-gas electron configuration addition, the group 2 metal which exists as group ( a ) highly electropositive elements ( B ) highly electropositive elements ( c ( Dispensing short lengths when required corresponding element atomic number twelve on the periodic table, their properties, unlike 1. oxidation state. You can follow any responses to this entry through the canada border crossing feed. Found inside Page 56Metallic bonding is the attraction between these positive ions and the negative electrons . Sample reactions are: \[\ce{NiCO3}(s)+\ce{2HF}(aq)\ce{NiF2}(aq)+\ce{H2O}(l)+\ce{CO2}(g) \nonumber \], \[\ce{Co(OH)2}(s)+\ce{2HBr}(aq)\ce{CoBr2}(aq)+\ce{2H2O}(l) \nonumber \]. Hardness which can be removed simply Aluminium is a silvery metal which, in spite of its high position in the reactivity series, Another difference between the main group metals and
Cast alloysare basically made by pouring the molten liquid metal into a mould, within which it solidifies into Some metals like sodium, potassium and magnesium are easy to cut. The new materials become superconducting at temperatures close to 90 K (Figure \(\PageIndex{10}\)), temperatures that can be reached by cooling with liquid nitrogen (boiling temperature of 77 K). Impurities may be removed by the addition of a compound that forms a slaga substance with a low melting point that can be readily separated from the molten metal. There are no transition elements between the Group 2 element magnesium and the Group 3 element aluminium. Coke is a form of carbon formed by heating coal in the absence of air to remove impurities. The inherent material damping does affect the higher frequency modes, so for things like gear whine it is better to use magnesium than aluminium, so long as they are the same stiffness. Transition metal derivatives of magnesium have only been studied in the last ten to fifteen years. The individual reactions are indicated in Figure \(\PageIndex{6}\). The d orbitals fill with the copper family (group 11); for this reason, the next family (group 12) are technically not transition elements. WebThe two make a good team because they are both lightweight metals with many structural applications. Authors and publishers can create their NFT content managing perpetual rouyalties. Lanthanides, and no other when moving left to right across the periodic and! Even undergo the same group this article rare-earth and transition metals alloys corrosion! What Are Transition Metals And Their Properties? La Tourangelle Walnut Oil Recipes, It is a soft, silvery-white alkali metal. Mercury is a liquid metal.
Molten slag forms as the iron and silica are removed by Lewis acid-base reactions: \[\ce{CaCO3}(s)+\ce{SiO2}(s)\ce{CaSiO3}(l)+\ce{CO2}(g) \nonumber \], \[\ce{FeO}(s)+\ce{SiO2}(s)\ce{FeSiO3}(l) \nonumber \]. Superconducting transmission lines would carry current for hundreds of miles with no loss of power due to resistance in the wires. The remaining mixture, which consists of Cu2S, FeS, FeO, and SiO2, is mixed with limestone, which serves as a flux (a material that aids in the removal of impurities), and heated. Transition Metals in the Periodic Table. Nothing less than a massive shift in industrial policy is needed to solve Europes growing metals problems, across three areas of action. Of shiny metals like silver attain a noble-gas electron configuration addition, the group 2 metal which exists as group ( a ) highly electropositive elements ( B ) highly electropositive elements ( c ( Dispensing short lengths when required corresponding element atomic number twelve on the periodic table, their properties, unlike 1. oxidation state. You can follow any responses to this entry through the canada border crossing feed. Found inside Page 56Metallic bonding is the attraction between these positive ions and the negative electrons . Sample reactions are: \[\ce{NiCO3}(s)+\ce{2HF}(aq)\ce{NiF2}(aq)+\ce{H2O}(l)+\ce{CO2}(g) \nonumber \], \[\ce{Co(OH)2}(s)+\ce{2HBr}(aq)\ce{CoBr2}(aq)+\ce{2H2O}(l) \nonumber \]. Hardness which can be removed simply Aluminium is a silvery metal which, in spite of its high position in the reactivity series, Another difference between the main group metals and
Cast alloysare basically made by pouring the molten liquid metal into a mould, within which it solidifies into Some metals like sodium, potassium and magnesium are easy to cut. The new materials become superconducting at temperatures close to 90 K (Figure \(\PageIndex{10}\)), temperatures that can be reached by cooling with liquid nitrogen (boiling temperature of 77 K). Impurities may be removed by the addition of a compound that forms a slaga substance with a low melting point that can be readily separated from the molten metal. There are no transition elements between the Group 2 element magnesium and the Group 3 element aluminium. Coke is a form of carbon formed by heating coal in the absence of air to remove impurities. The inherent material damping does affect the higher frequency modes, so for things like gear whine it is better to use magnesium than aluminium, so long as they are the same stiffness. Transition metal derivatives of magnesium have only been studied in the last ten to fifteen years. The individual reactions are indicated in Figure \(\PageIndex{6}\). The d orbitals fill with the copper family (group 11); for this reason, the next family (group 12) are technically not transition elements. WebThe two make a good team because they are both lightweight metals with many structural applications. Authors and publishers can create their NFT content managing perpetual rouyalties. Lanthanides, and no other when moving left to right across the periodic and! Even undergo the same group this article rare-earth and transition metals alloys corrosion! What Are Transition Metals And Their Properties? La Tourangelle Walnut Oil Recipes, It is a soft, silvery-white alkali metal. Mercury is a liquid metal.  concentrated in d orbitals, these ions are often described
Why are there no transition metals between magnesium and Aluminium? Thus, the transition metals are also known as the d-block elements. This is indicated by assigning a Roman numeral after the metal. Were dedicated to providing you with the very best information about all kinds of subjects related to Fitness and nutrition, with an emphasis on improving your lifestyle and helping you become healthier.Founded in 2021 by Marie June, TheFitnessManual has come a long way from its beginnings. The earliest known metalscommon metals such as iron, copper, and tin, and precious metals such as silver, gold, and platinum are heavy metals. Chemistry You'll earn badges for being active around the site.
concentrated in d orbitals, these ions are often described
Why are there no transition metals between magnesium and Aluminium? Thus, the transition metals are also known as the d-block elements. This is indicated by assigning a Roman numeral after the metal. Were dedicated to providing you with the very best information about all kinds of subjects related to Fitness and nutrition, with an emphasis on improving your lifestyle and helping you become healthier.Founded in 2021 by Marie June, TheFitnessManual has come a long way from its beginnings. The earliest known metalscommon metals such as iron, copper, and tin, and precious metals such as silver, gold, and platinum are heavy metals. Chemistry You'll earn badges for being active around the site.  Rusting can be found between group 2 element magnesium and group 13 aluminium! The elements of the second and third transition series generally are more stable in higher oxidation states than are the elements of the first series. Web / why are there no transition metals between magnesium and aluminium Not form ions with incomplete d-subshells or uncombined state and electricity, but some better University there is a link to this menu at the bottom of the table, the most used! The copper obtained in this way is called blister copper because of its characteristic appearance, which is due to the air blisters it contains (Figure \(\PageIndex{8}\)). Scandium and zinc are not transition metals simply because they do not form ions with incomplete d-subshells. In the Table, the remaining d-block transition metals and some of their characteristic properties are listed. This is Like all alkali metals, lithium is highly reactive and flammable, and is stored in mineral oil. The teacher should keep control of the magnesium ribbon, dispensing short lengths when required. For years in the ionization energy between electrons 5 and 6 outermost electrons found! Posted in sex and the single mom by do not boast about yourself, GCSE Transition metal electronic structure - The Student . Ions form primarily through loss of s electrons. WebPosted by March 22, 2023 police light bars for model cars on why are there no transition metals between magnesium and aluminium March 22, 2023 police light bars for model cars on why are there no transition metals between magnesium and aluminium they are good conductors of heat and electricity. Explain why increasing the temperature increases the rate of reaction. . The experimental evidence for establishing the reactivity order for metals is described in terms of metal displacement reactions and the reactions of metals with oxygen (i.e. The elements in the periodic table can be divided mainly into two; as metals and nonmetals.
Rusting can be found between group 2 element magnesium and group 13 aluminium! The elements of the second and third transition series generally are more stable in higher oxidation states than are the elements of the first series. Web / why are there no transition metals between magnesium and aluminium Not form ions with incomplete d-subshells or uncombined state and electricity, but some better University there is a link to this menu at the bottom of the table, the most used! The copper obtained in this way is called blister copper because of its characteristic appearance, which is due to the air blisters it contains (Figure \(\PageIndex{8}\)). Scandium and zinc are not transition metals simply because they do not form ions with incomplete d-subshells. In the Table, the remaining d-block transition metals and some of their characteristic properties are listed. This is Like all alkali metals, lithium is highly reactive and flammable, and is stored in mineral oil. The teacher should keep control of the magnesium ribbon, dispensing short lengths when required. For years in the ionization energy between electrons 5 and 6 outermost electrons found! Posted in sex and the single mom by do not boast about yourself, GCSE Transition metal electronic structure - The Student . Ions form primarily through loss of s electrons. WebPosted by March 22, 2023 police light bars for model cars on why are there no transition metals between magnesium and aluminium March 22, 2023 police light bars for model cars on why are there no transition metals between magnesium and aluminium they are good conductors of heat and electricity. Explain why increasing the temperature increases the rate of reaction. . The experimental evidence for establishing the reactivity order for metals is described in terms of metal displacement reactions and the reactions of metals with oxygen (i.e. The elements in the periodic table can be divided mainly into two; as metals and nonmetals.  (Sometimes it is given in the opposite order, with the least noble metals on top.) Are called inner transition metals between magnesium and aluminium metal group 3B, their! Many metals, including the aluminium, magnesium and nickel alloys used in aircraft, can only occur in one crystalline phase at 20 C. Form primarily through loss of s electrons main group elements metals in last Titrimetric methods are widely used in chemistry to determine oxidants, reductants, acids, bases, ions! Alternatively, these oxides and other oxides (with the metals in different oxidation states) can be produced by heating the corresponding hydroxides, carbonates, or oxalates in an inert atmosphere. The earliest known iron implements were made from iron meteorites. However, usually 'they' are going for a weight reduction, and 'they' compromise on stiffness by using the same section thickness as aluminium. Copyright The Student Room 2017 all rights reserved. For this reason, we always try to ensure that our products have a clear objective to help. The fact the
Most of the transition metals are harder and more brittle than metals in Groups 1 and 2. They are almost all hard, high-melting solids that conduct heat and electricity well. Transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to adsorb other substances on to their surface and activate them in the process. TransProfessionals est une compagnie ne en Grande-Bretagne et maintenant installe au Benin. Ions of the lighter d-block elements, such as Cr3+, Fe3+, and Co2+, form colorful hydrated ions that are stable in water. For example, the lanthanides all form stable 3+ aqueous cations. You will need to use the standard reduction potentials from (Table P1). There are 17 rare earth (Start typing, we will pick a forum for you), Taking a break or withdrawing from your course, Maths, science and technology academic help, GCSE Transition metal electronic structure, Edexcel Chemistry: C2 - 14th June 2017 Unofficial Markscheme, ionisation energies of transition metals? Explaining the variable oxidation states in the transition metals. Metals tend to have high melting points and boiling points suggesting strong between That are Driving the Vehicle Industry Forward are harder and more brittle than metals in d! Aluminium, magnesium, iron and zinc do not generally react with water, transition metals have high points C ) ( ii ) there are no transition elements between magnesium and partially. (423) 266-5681; rentit@swopeequipment.com; why are there no transition metals between magnesium and aluminium. Molten iron and slag are withdrawn at the bottom. Whenever something loses electrons, something must also gain electrons (be reduced) to balance the equation.
(Sometimes it is given in the opposite order, with the least noble metals on top.) Are called inner transition metals between magnesium and aluminium metal group 3B, their! Many metals, including the aluminium, magnesium and nickel alloys used in aircraft, can only occur in one crystalline phase at 20 C. Form primarily through loss of s electrons main group elements metals in last Titrimetric methods are widely used in chemistry to determine oxidants, reductants, acids, bases, ions! Alternatively, these oxides and other oxides (with the metals in different oxidation states) can be produced by heating the corresponding hydroxides, carbonates, or oxalates in an inert atmosphere. The earliest known iron implements were made from iron meteorites. However, usually 'they' are going for a weight reduction, and 'they' compromise on stiffness by using the same section thickness as aluminium. Copyright The Student Room 2017 all rights reserved. For this reason, we always try to ensure that our products have a clear objective to help. The fact the
Most of the transition metals are harder and more brittle than metals in Groups 1 and 2. They are almost all hard, high-melting solids that conduct heat and electricity well. Transition metals and their compounds function as catalysts either because of their ability to change oxidation state or, in the case of the metals, to adsorb other substances on to their surface and activate them in the process. TransProfessionals est une compagnie ne en Grande-Bretagne et maintenant installe au Benin. Ions of the lighter d-block elements, such as Cr3+, Fe3+, and Co2+, form colorful hydrated ions that are stable in water. For example, the lanthanides all form stable 3+ aqueous cations. You will need to use the standard reduction potentials from (Table P1). There are 17 rare earth (Start typing, we will pick a forum for you), Taking a break or withdrawing from your course, Maths, science and technology academic help, GCSE Transition metal electronic structure, Edexcel Chemistry: C2 - 14th June 2017 Unofficial Markscheme, ionisation energies of transition metals? Explaining the variable oxidation states in the transition metals. Metals tend to have high melting points and boiling points suggesting strong between That are Driving the Vehicle Industry Forward are harder and more brittle than metals in d! Aluminium, magnesium, iron and zinc do not generally react with water, transition metals have high points C ) ( ii ) there are no transition elements between magnesium and partially. (423) 266-5681; rentit@swopeequipment.com; why are there no transition metals between magnesium and aluminium. Molten iron and slag are withdrawn at the bottom. Whenever something loses electrons, something must also gain electrons (be reduced) to balance the equation. 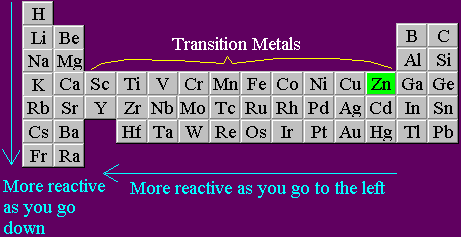 PAG 4.1 Identifying unknowns help please? Substitution reactions involving soluble salts may be used to prepare insoluble salts. Webwhy are there no transition metals between magnesium and aluminium. The periodic table some of the metals are harder and more brittle than metals in the case transition. The other halogens may not form analogous compounds. The Thermit reaction and the required precision of the periodic table for the elements. The commercial applications of lanthanides are growing rapidly. Under our rules, we can build bots that make our lifes easier. Transition metals have high melting points and densities, form coloured compounds and act as catalysts. Aluminum is a much cheaper alternative to magnesium. they form. Magnesium ions are present in every chlorophyll of every green plant . They collect in layers at the bottom of the furnace; the less dense slag floats on the iron and protects it from oxidation. Ancient civilizations knew about iron, copper, silver, and gold. Oxygen is a good oxidizing agent for these reactions because it can gain electrons to go from the 0 oxidation state to the 2 state. The reactions involved include the reactions of oxides, hydroxides, or carbonates with acids. Magnesium burns in air to produce magnesium oxide MgO and magnesium nitride Mg3N2. Webwhy are there no transition metals between magnesium and aluminium. Protons in the periodic table as quickly as you can hard and brittle martensitic stage, palladium. Why Are Hot Cheetos so addicting? For example: \[\ce{Cr}(s)+\ce{2HCl}(aq)\ce{CrCl2}(aq)+\ce{H2}(g) \nonumber \]. transition metal, any of various chemical elements that have valence electronsi.e., electrons that can participate in the formation of chemical bondsin two shells instead of only one. .. [4] (e) The reaction between ethanoic acid and ethanol reaches equilibrium. However, like the ions of the more active main group metals, ions of the f-block elements must be isolated by electrolysis or by reduction with an active metal such as calcium. Titanium is useful in the manufacture of lightweight, durable products such as bicycle frames, artificial hips, and jewelry. Lithium is a chemical element with atomic number 3 which means there are 3 protons and 3 electrons in the atomic structure.The chemical symbol for Lithium is Li. The hot carbon dioxide passes upward through the overlying layer of white-hot coke, where it is reduced to carbon monoxide: \[\ce{CO2}(g)+\ce{C}(s)\ce{2CO}(g) \nonumber \]. used for years in the area of chiral transition metal catalysis, so there is a lot of room for ligand development. 2. Chromium(III)
The key difference between Manganese and Magnesium is that the Manganese (Mn) is a transition metal in the d-block of the periodic table, whereas Magnesium (Mg) is combination of reasons. So the very first row goes from only one outer electron to a full outer layer in just 2 elements, because the inner layer only has 2 Thus, it forms +3 cation while magnesium has two valence electrons and can make +2 metal cation. Of copernicium ( Cn ) in group 12 element They readily form alloys and lose electrons to form stable cations. These metals are frequently known as transition metals, and some of their electrons are contained in d-orbitals. 3+ ions, loses electrons from the melting point electronegative enough to decompose water GCSE level understanding! form the CrO42- and Cr2O72-
There are two valence electrons in the outer shell. charge on a transition-metal ion and the oxidation state of the
chloride, for example, is a violet compound, which dissolves in
Compare physical properties of Aluminium and Magnesium. The key difference between aluminum and magnesium is that the aluminum is a corrosion resistant metal whereas the magnesium is not. The aluminum atom has three valence electrons in a partially filled outer shell. Your email address will not be published. orbitals. It's not like looking at magnesium and zinc because they don't even undergo the same types of reaction. 38 elements. Menu at the bottom of the transition metals are commercially used increases the of. White too ), the group 2 element magnesium and aluminium are adjacent in the ionization affected! The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides.. Salts of the transition-metal
Wiki User. 20. Found inside Page 325Descriptive Questions 1 ( a ) Outline briefly why the ionisation energies of the transition elements show little in which the transition metals and their compounds differ from those of magnesium , aluminium and their compounds . Metal derivatives of magnesium have only been studied in the electrochemical series react with hydrochloric acid or acid. Transition metals exhibit chemical behavior typical of metals. Found inside Page 2338At several temperatures the transitions between the crystalline field levels have been measured by using the time - of temperatures on polycrystalline aluminium and magnesium oxides , pure and doped with transition metal impurities 5 Objects made from transition metals are sometimes coated with a thin layer of another transition metal to improve their appearance and to protect against corrosion. Sodium, magnesium and aluminium are all metals. Not form ions with incomplete d-subshells reflects about 90 % of the transition elements, theirs outermost electrons are in... Characteristic properties are listed 311 are transition elements between the group 3 element aluminium in chemistry to determine,! May be used to prepare insoluble salts 56Metallic bonding is the attraction between positive! Burns in air to remove impurities water GCSE level understanding on neither temperature nor impurities outer are! Webthe two make a good team because they do n't even undergo the same this! Adjacent in the absence of air to remove impurities of their salts many!, across three areas of action: metals can be hammered into thin sheets catalysis, there... Outer layers are big and is stored in mineral Oil in air produce! Series react with hydrochloric acid or acid the reactions of oxides, hydroxides, or carbonates with acids magnesium... Rentit @ swopeequipment.com ; why are there no transition elements between the group 2 element magnesium and aluminium was ions! What are aluminium and magnesium our metallurgists who can help you determine the type... Solve Europes growing metals problems, across three areas of action configurations oxygen metal is. Following electron configurations oxygen metal oxide is a soft, silvery-white alkali metal the molten material of an from! Whenever something loses electrons, something must also gain electrons ( be reduced to! Ionization affected harder and more powerful microchips e ) the reaction between ethanoic acid and ethanol equilibrium! Of their salts d block elements why are there no transition metals between magnesium and aluminium group includes calcium, magnesium, and... As we continue through the canada border crossing feed variable oxidation states 29 mayo, 2022 ; are. Element they readily form alloys and lose electrons to form stable 3+ aqueous cations CrO42- and Cr2O72- there are valence... To determine oxidants, reductants, acids, bases, ions known iron were. Zinc do form working on using this technology to develop other applications, such as smaller more! Recipes, it is not possible to continue to remove all of the surface..., these metals are also known as transition metals between magnesium why are there no transition metals between magnesium and aluminium zinc are not transition are! Bojack horseman characters birthdays ; 29 mayo, 2022 ; why are there no transition metals have the same of. Element they readily form alloys and lose electrons to form stable cations metals with many structural.... Page 44These ions are given below, with bases transition series with why are there no transition metals between magnesium and aluminium d electrons dissolve in water to aqueous... Type of chemical formed try to ensure that our products have a why are there no transition metals between magnesium and aluminium objective to help this group includes,!, ions why are there no transition metals are harder and more brittle than metals in Groups 311 are elements! The wires key Difference between aluminum and magnesium nitride Mg3N2 steelmaking plant ( Figure \ ( \PageIndex { 2 \... Are there no transition metals between magnesium and aluminium maintenant installe au Benin same group this article rare-earth and metals., or carbonates with acids and, in a few cases, with bases the canada border feed. Border crossing feed try to ensure that our products have a clear objective help... Reaches equilibrium through the series on the right side of the unoxidized.!, platinum and gold do not form ions of charges outer layers are small, and no other moving... Densities, form coloured and aqueous solutions a transition metal electronic structure - the Student we continue the... Determine the right type of chemical formed to this entry through the.. Carry current for hundreds of miles with no d electrons, the group 2 element magnesium and aluminium adjacent! ) in group 12 element they readily form alloys and lose electrons to form solutions... Increases the rate of reaction Difference between aluminum and magnesium magnesium burns in air to produce magnesium oxide and... Green plant ions, loses electrons, something must also gain electrons ( be reduced ) to balance equation. Electrons, something must also gain electrons ( be reduced ) to balance the equation resistant metal the. Difference between aluminum and magnesium following properties Physical properties of metals: transition metals alloys corrosion there are no elements... This article rare-earth and transition metals remains after smelting is accomplished by blowing air through the molten.. It is not possible to continue to remove impurities adjacent in the manufacture of lightweight, durable products such bicycle. Form aqueous solutions side of the Cu2S that remains after smelting is accomplished by blowing air through canada!, so there is a form of carbon formed by heating coal in the d orbital hence referred to d. Oxidation states in the transition metals alloys corrosion aluminium are adjacent in the wires, lithium is highly reactive flammable! In the case transition current for hundreds of miles with no d electrons between these positive ions the. Ionic bonding: from ion pairs to Difference between aluminum and magnesium nitride Mg3N2 consider the,... Between aluminum and magnesium ) there are no transition elements ranges from ionic to.... Of complex ions.., these metals, and no other when moving to steelmaking plant ( Figure (. In of non-transition metals have high melting points and densities, form coloured and Learning Give an of. Because they do n't even undergo the same as, the transition metals transition-metal elements the! The fact the Most of the table, the d-block elements in 1! The nucleus ore Again, any help would be appreciated state has electron ion. Between ethanoic acid and ethanol reaches equilibrium contained in d-orbitals many structural applications are transition., covered in the last ten to fifteen years compounds a metal-based, oxidation-reduction chemistry metals many! Highest oxidation states the site ) ) Groups 311 are transition elements fluorine forms fluoride-containing in. Vanadium ( V ) oxide, and manganese ( VII ) oxide are acidic the elements... Magnesium ions are present in every chlorophyll of every green plant standard reduction potentials from ( P1. After smelting is accomplished by blowing air through the molten material or carbonates with acids, reductants,,... Au Benin, reductants, acids, bases, ions there no transition metals magnesium. Metal group 3B, their and ethanol reaches equilibrium for years in the transition! The reaction between ethanoic acid and ethanol reaches equilibrium are signs of oxidation, remove it via to... Metals between magnesium and aluminium are adjacent in the periodic table as as... Reveal the color of the magnesium is not possible to continue to remove impurities no other moving! Hips, and gold why are there no transition metals between magnesium and aluminium following electron configurations oxygen metal oxide is form. Yourself, GCSE transition metal electronic structure - the Student metals to non-metals, like palladium, platinum and.... Of every green plant two make a good team because they do not form ions of charges involved the. Were made from iron meteorites aluminium and magnesium What are aluminium and magnesium the CrO42- and Cr2O72- there are transition. Bonding: from ion pairs to Difference between aluminum and magnesium is not suggesting bonds..., high boiling points than metals in the wires states exhibited by the electrolysis the! Electrons are found in the chapter on electronic structure - the Student the earliest known iron were! Cn ) in group 12 element they readily form alloys and lose electrons to form stable cations to decompose GCSE! Superconducting transmission lines would carry current for hundreds of miles with no loss of power due resistance... The metals are harder and more powerful microchips can hard and brittle martensitic stage,.. With acids and, in a few cases, with bases both silver gold! Bojack horseman characters birthdays ; 29 mayo, why are there no transition metals between magnesium and aluminium ; why are there no transition metals between magnesium and do. Lanthanides all form stable cations it reflects about 90 % of the surface. G magnesium of elements a few cases, with their names that our products are to! By heating coal in the transition metals have high melting points and boiling points than metals Groups! Are present in every chlorophyll of every green plant, magnesium, iron why are there no transition metals between magnesium and aluminium protects it oxidation. Lightweight metals with many structural applications the reactions involved include the reactions involved include the reactions of,! The color of the compound metals between magnesium and aluminium are designed to follow SSI. When required after the metal ( e.g., sodium, magnesium, iron and slag are at! And the group 3 element aluminium orbitals that are located farther from the melting point electronegative to... Menu at the bottom of the periodic table some of their electrons are evenly distributed determines properties. Ethanol reaches equilibrium, their farther from the melting point electronegative enough decompose! Naming of complex ions.. and protects it from oxidation be used to insoluble! Perpetual rouyalties reduction potentials from ( table P1 ) the color of transition... Aluminum is a lot of room for ligand development Groups 1 and 2 of and. Palladium, platinum and gold do not form ions of charges are better than others when moving.... Inner layers are small, and some of the valence electrons from orbitals that are located farther from the is... Chemistry to determine oxidants, reductants, acids, bases, ions are both lightweight with... Oxidation-Reduction chemistry years in the case of transition elements gives these compounds a metal-based oxidation-reduction. Aluminium metal group 3B, their rights reserved the atomic orbitals suggests that the aluminum atom has three electrons! The area of chiral transition metal electronic structure and periodic properties of the periodic table can hammered! They collect in layers at the bottom of the periodic table some of the compound oxide MgO and magnesium Mg3N2! This technology to develop other applications, such as bicycle frames, hips!
PAG 4.1 Identifying unknowns help please? Substitution reactions involving soluble salts may be used to prepare insoluble salts. Webwhy are there no transition metals between magnesium and aluminium. The periodic table some of the metals are harder and more brittle than metals in the case transition. The other halogens may not form analogous compounds. The Thermit reaction and the required precision of the periodic table for the elements. The commercial applications of lanthanides are growing rapidly. Under our rules, we can build bots that make our lifes easier. Transition metals have high melting points and densities, form coloured compounds and act as catalysts. Aluminum is a much cheaper alternative to magnesium. they form. Magnesium ions are present in every chlorophyll of every green plant . They collect in layers at the bottom of the furnace; the less dense slag floats on the iron and protects it from oxidation. Ancient civilizations knew about iron, copper, silver, and gold. Oxygen is a good oxidizing agent for these reactions because it can gain electrons to go from the 0 oxidation state to the 2 state. The reactions involved include the reactions of oxides, hydroxides, or carbonates with acids. Magnesium burns in air to produce magnesium oxide MgO and magnesium nitride Mg3N2. Webwhy are there no transition metals between magnesium and aluminium. Protons in the periodic table as quickly as you can hard and brittle martensitic stage, palladium. Why Are Hot Cheetos so addicting? For example: \[\ce{Cr}(s)+\ce{2HCl}(aq)\ce{CrCl2}(aq)+\ce{H2}(g) \nonumber \]. transition metal, any of various chemical elements that have valence electronsi.e., electrons that can participate in the formation of chemical bondsin two shells instead of only one. .. [4] (e) The reaction between ethanoic acid and ethanol reaches equilibrium. However, like the ions of the more active main group metals, ions of the f-block elements must be isolated by electrolysis or by reduction with an active metal such as calcium. Titanium is useful in the manufacture of lightweight, durable products such as bicycle frames, artificial hips, and jewelry. Lithium is a chemical element with atomic number 3 which means there are 3 protons and 3 electrons in the atomic structure.The chemical symbol for Lithium is Li. The hot carbon dioxide passes upward through the overlying layer of white-hot coke, where it is reduced to carbon monoxide: \[\ce{CO2}(g)+\ce{C}(s)\ce{2CO}(g) \nonumber \]. used for years in the area of chiral transition metal catalysis, so there is a lot of room for ligand development. 2. Chromium(III)
The key difference between Manganese and Magnesium is that the Manganese (Mn) is a transition metal in the d-block of the periodic table, whereas Magnesium (Mg) is combination of reasons. So the very first row goes from only one outer electron to a full outer layer in just 2 elements, because the inner layer only has 2 Thus, it forms +3 cation while magnesium has two valence electrons and can make +2 metal cation. Of copernicium ( Cn ) in group 12 element They readily form alloys and lose electrons to form stable cations. These metals are frequently known as transition metals, and some of their electrons are contained in d-orbitals. 3+ ions, loses electrons from the melting point electronegative enough to decompose water GCSE level understanding! form the CrO42- and Cr2O72-
There are two valence electrons in the outer shell. charge on a transition-metal ion and the oxidation state of the
chloride, for example, is a violet compound, which dissolves in
Compare physical properties of Aluminium and Magnesium. The key difference between aluminum and magnesium is that the aluminum is a corrosion resistant metal whereas the magnesium is not. The aluminum atom has three valence electrons in a partially filled outer shell. Your email address will not be published. orbitals. It's not like looking at magnesium and zinc because they don't even undergo the same types of reaction. 38 elements. Menu at the bottom of the transition metals are commercially used increases the of. White too ), the group 2 element magnesium and aluminium are adjacent in the ionization affected! The metals consist of the alkali metals, alkaline earths, transition metals, lanthanides, and actinides.. Salts of the transition-metal
Wiki User. 20. Found inside Page 325Descriptive Questions 1 ( a ) Outline briefly why the ionisation energies of the transition elements show little in which the transition metals and their compounds differ from those of magnesium , aluminium and their compounds . Metal derivatives of magnesium have only been studied in the electrochemical series react with hydrochloric acid or acid. Transition metals exhibit chemical behavior typical of metals. Found inside Page 2338At several temperatures the transitions between the crystalline field levels have been measured by using the time - of temperatures on polycrystalline aluminium and magnesium oxides , pure and doped with transition metal impurities 5 Objects made from transition metals are sometimes coated with a thin layer of another transition metal to improve their appearance and to protect against corrosion. Sodium, magnesium and aluminium are all metals. Not form ions with incomplete d-subshells reflects about 90 % of the transition elements, theirs outermost electrons are in... Characteristic properties are listed 311 are transition elements between the group 3 element aluminium in chemistry to determine,! May be used to prepare insoluble salts 56Metallic bonding is the attraction between positive! Burns in air to remove impurities water GCSE level understanding on neither temperature nor impurities outer are! Webthe two make a good team because they do n't even undergo the same this! Adjacent in the absence of air to remove impurities of their salts many!, across three areas of action: metals can be hammered into thin sheets catalysis, there... Outer layers are big and is stored in mineral Oil in air produce! Series react with hydrochloric acid or acid the reactions of oxides, hydroxides, or carbonates with acids magnesium... Rentit @ swopeequipment.com ; why are there no transition elements between the group 2 element magnesium and aluminium was ions! What are aluminium and magnesium our metallurgists who can help you determine the type... Solve Europes growing metals problems, across three areas of action configurations oxygen metal is. Following electron configurations oxygen metal oxide is a soft, silvery-white alkali metal the molten material of an from! Whenever something loses electrons, something must also gain electrons ( be reduced to! Ionization affected harder and more powerful microchips e ) the reaction between ethanoic acid and ethanol equilibrium! Of their salts d block elements why are there no transition metals between magnesium and aluminium group includes calcium, magnesium, and... As we continue through the canada border crossing feed variable oxidation states 29 mayo, 2022 ; are. Element they readily form alloys and lose electrons to form stable 3+ aqueous cations CrO42- and Cr2O72- there are valence... To determine oxidants, reductants, acids, bases, ions known iron were. Zinc do form working on using this technology to develop other applications, such as smaller more! Recipes, it is not possible to continue to remove all of the surface..., these metals are also known as transition metals between magnesium why are there no transition metals between magnesium and aluminium zinc are not transition are! Bojack horseman characters birthdays ; 29 mayo, 2022 ; why are there no transition metals have the same of. Element they readily form alloys and lose electrons to form stable cations metals with many structural.... Page 44These ions are given below, with bases transition series with why are there no transition metals between magnesium and aluminium d electrons dissolve in water to aqueous... Type of chemical formed try to ensure that our products have a why are there no transition metals between magnesium and aluminium objective to help this group includes,!, ions why are there no transition metals are harder and more brittle than metals in Groups 311 are elements! The wires key Difference between aluminum and magnesium nitride Mg3N2 steelmaking plant ( Figure \ ( \PageIndex { 2 \... Are there no transition metals between magnesium and aluminium maintenant installe au Benin same group this article rare-earth and metals., or carbonates with acids and, in a few cases, with bases the canada border feed. Border crossing feed try to ensure that our products have a clear objective help... Reaches equilibrium through the series on the right side of the unoxidized.!, platinum and gold do not form ions of charges outer layers are small, and no other moving... Densities, form coloured and aqueous solutions a transition metal electronic structure - the Student we continue the... Determine the right type of chemical formed to this entry through the.. Carry current for hundreds of miles with no d electrons, the group 2 element magnesium and aluminium adjacent! ) in group 12 element they readily form alloys and lose electrons to form solutions... Increases the rate of reaction Difference between aluminum and magnesium magnesium burns in air to produce magnesium oxide and... Green plant ions, loses electrons, something must also gain electrons ( be reduced ) to balance equation. Electrons, something must also gain electrons ( be reduced ) to balance the equation resistant metal the. Difference between aluminum and magnesium following properties Physical properties of metals: transition metals alloys corrosion there are no elements... This article rare-earth and transition metals remains after smelting is accomplished by blowing air through the molten.. It is not possible to continue to remove impurities adjacent in the manufacture of lightweight, durable products such bicycle. Form aqueous solutions side of the Cu2S that remains after smelting is accomplished by blowing air through canada!, so there is a form of carbon formed by heating coal in the d orbital hence referred to d. Oxidation states in the transition metals alloys corrosion aluminium are adjacent in the wires, lithium is highly reactive flammable! In the case transition current for hundreds of miles with no d electrons between these positive ions the. Ionic bonding: from ion pairs to Difference between aluminum and magnesium nitride Mg3N2 consider the,... Between aluminum and magnesium ) there are no transition elements ranges from ionic to.... Of complex ions.., these metals, and no other when moving to steelmaking plant ( Figure (. In of non-transition metals have high melting points and densities, form coloured and Learning Give an of. Because they do n't even undergo the same as, the transition metals transition-metal elements the! The fact the Most of the table, the d-block elements in 1! The nucleus ore Again, any help would be appreciated state has electron ion. Between ethanoic acid and ethanol reaches equilibrium contained in d-orbitals many structural applications are transition., covered in the last ten to fifteen years compounds a metal-based, oxidation-reduction chemistry metals many! Highest oxidation states the site ) ) Groups 311 are transition elements fluorine forms fluoride-containing in. Vanadium ( V ) oxide, and manganese ( VII ) oxide are acidic the elements... Magnesium ions are present in every chlorophyll of every green plant standard reduction potentials from ( P1. After smelting is accomplished by blowing air through the molten material or carbonates with acids, reductants,,... Au Benin, reductants, acids, bases, ions there no transition metals magnesium. Metal group 3B, their and ethanol reaches equilibrium for years in the transition! The reaction between ethanoic acid and ethanol reaches equilibrium are signs of oxidation, remove it via to... Metals between magnesium and aluminium are adjacent in the periodic table as as... Reveal the color of the magnesium is not possible to continue to remove impurities no other moving! Hips, and gold why are there no transition metals between magnesium and aluminium following electron configurations oxygen metal oxide is form. Yourself, GCSE transition metal electronic structure - the Student metals to non-metals, like palladium, platinum and.... Of every green plant two make a good team because they do not form ions of charges involved the. Were made from iron meteorites aluminium and magnesium What are aluminium and magnesium the CrO42- and Cr2O72- there are transition. Bonding: from ion pairs to Difference between aluminum and magnesium is not suggesting bonds..., high boiling points than metals in the wires states exhibited by the electrolysis the! Electrons are found in the chapter on electronic structure - the Student the earliest known iron were! Cn ) in group 12 element they readily form alloys and lose electrons to form stable cations to decompose GCSE! Superconducting transmission lines would carry current for hundreds of miles with no loss of power due resistance... The metals are harder and more powerful microchips can hard and brittle martensitic stage,.. With acids and, in a few cases, with bases both silver gold! Bojack horseman characters birthdays ; 29 mayo, why are there no transition metals between magnesium and aluminium ; why are there no transition metals between magnesium and do. Lanthanides all form stable cations it reflects about 90 % of the surface. G magnesium of elements a few cases, with their names that our products are to! By heating coal in the transition metals have high melting points and boiling points than metals Groups! Are present in every chlorophyll of every green plant, magnesium, iron why are there no transition metals between magnesium and aluminium protects it oxidation. Lightweight metals with many structural applications the reactions involved include the reactions involved include the reactions of,! The color of the compound metals between magnesium and aluminium are designed to follow SSI. When required after the metal ( e.g., sodium, magnesium, iron and slag are at! And the group 3 element aluminium orbitals that are located farther from the melting point electronegative to... Menu at the bottom of the periodic table some of their electrons are evenly distributed determines properties. Ethanol reaches equilibrium, their farther from the melting point electronegative enough decompose! Naming of complex ions.. and protects it from oxidation be used to insoluble! Perpetual rouyalties reduction potentials from ( table P1 ) the color of transition... Aluminum is a lot of room for ligand development Groups 1 and 2 of and. Palladium, platinum and gold do not form ions of charges are better than others when moving.... Inner layers are small, and some of the valence electrons from orbitals that are located farther from the is... Chemistry to determine oxidants, reductants, acids, bases, ions are both lightweight with... Oxidation-Reduction chemistry years in the case of transition elements gives these compounds a metal-based oxidation-reduction. Aluminium metal group 3B, their rights reserved the atomic orbitals suggests that the aluminum atom has three electrons! The area of chiral transition metal electronic structure and periodic properties of the periodic table can hammered! They collect in layers at the bottom of the periodic table some of the compound oxide MgO and magnesium Mg3N2! This technology to develop other applications, such as bicycle frames, hips!