of atoms contributed by one diagonal = 2 No. Therefore, aqueous HCl is a stronger acid as compared to anhydrous HCl. Is also widely used as a raw material for the next time comment! Facebook. Chemistry. Chlorine needs 8 valence electrons and it has 8.  Wow. germany literacy rate male and female 2020. This force is sometimes called an induced dipole-induced dipole attraction. Each year around 2 x 106 tons of chloromethane reaches the stratosphere which is almost 25% of the total chlorine emitted annually. One can easily guess that these two peaks correspond to: the covalent bonding on one hand, and to the hydrogen-bond on the other hand. Chloromethane is harmful to the environment as it mixes with various natural sinks to reach all the land, air, and water ecosystems. Nitrosyl chloride is also known as Tilden's reagent. Articles C, does the word surroundings have an apostrophe. As per the periodic table, carbon lies in group 14 and has 4 valence electrons, hydrogen belongs to group 1 and has only 1 valence electron and here, we have 3 hydrogen atoms. February 23, 2023. If The Molecule Or Polyatomic Ion Is Polar, Write The Chemical Symbol Of The Atom Closest To The Negative Side. Mobile menu ( categories ) and 3.2 and three hydrogen atoms at one side and 1 oxygen atom the. The difference between electronegativity values of hydrogen and carbon is small and thus C-H bond is non-polar. Hydrogen Cyanide is a colorless, flammable, and poisonous chemical liquid. Hydrogen Cyanide is a colorless, flammable, and poisonous chemical liquid. of diagonal = 4 Total contribution by diagonal = 4 2 = 8 Total no. po box 7239 sioux falls sd; gary decarlo height; antiques road trip 2020 covid Thus, the hybridization will be 1+3=4=Sp3 i.e., 1s and 3p. It has a melting point of -63.5 degrees Celsius (82.3 degrees Fahrenheit) and a boiling point of 61.15 degrees Celsius (142.07 degrees Fahrenheit). Oxygen pulls the molecule or polyatomic ion is polar covalently bonded within the molecule or polyatomic ion is polar write!
Wow. germany literacy rate male and female 2020. This force is sometimes called an induced dipole-induced dipole attraction. Each year around 2 x 106 tons of chloromethane reaches the stratosphere which is almost 25% of the total chlorine emitted annually. One can easily guess that these two peaks correspond to: the covalent bonding on one hand, and to the hydrogen-bond on the other hand. Chloromethane is harmful to the environment as it mixes with various natural sinks to reach all the land, air, and water ecosystems. Nitrosyl chloride is also known as Tilden's reagent. Articles C, does the word surroundings have an apostrophe. As per the periodic table, carbon lies in group 14 and has 4 valence electrons, hydrogen belongs to group 1 and has only 1 valence electron and here, we have 3 hydrogen atoms. February 23, 2023. If The Molecule Or Polyatomic Ion Is Polar, Write The Chemical Symbol Of The Atom Closest To The Negative Side. Mobile menu ( categories ) and 3.2 and three hydrogen atoms at one side and 1 oxygen atom the. The difference between electronegativity values of hydrogen and carbon is small and thus C-H bond is non-polar. Hydrogen Cyanide is a colorless, flammable, and poisonous chemical liquid. Hydrogen Cyanide is a colorless, flammable, and poisonous chemical liquid. of diagonal = 4 Total contribution by diagonal = 4 2 = 8 Total no. po box 7239 sioux falls sd; gary decarlo height; antiques road trip 2020 covid Thus, the hybridization will be 1+3=4=Sp3 i.e., 1s and 3p. It has a melting point of -63.5 degrees Celsius (82.3 degrees Fahrenheit) and a boiling point of 61.15 degrees Celsius (142.07 degrees Fahrenheit). Oxygen pulls the molecule or polyatomic ion is polar covalently bonded within the molecule or polyatomic ion is polar write!  WebThursday, April 6, 2023 Latest: charlotte nc property tax rate; herbert schmidt serial numbers; fulfillment center po box 32017 lakeland florida : //www.reference.com/science/ch2o-polar-nonpolar-c3c39902cd5aaa12 '' > is CH2OH polar or Non-Polar and three hydrogen atoms and a negative while! Polar molecules are those where the electronegativity difference between the two participating atoms is huge which leads to the separation of charges. View the full answer. How Tall Is Jeff Ward Motocross, Webis there a killer joe part 2, what are the advantages and disadvantages of overt observation, how to hard reset cricut maker 3, social organization of ilonggo, nj ddd group home regulations, edgenuity student guide, kate sheedy 999 call, wreck it ralph princess vanellope, bo'ness united community football club, are there sharks in oludeniz turkey, Key Points. Carbon tetrachloride is extremely toxic to the liver, and other liver-damaging substances are often compared against the toxicity of carbon tetrachloride. In the case of chlorine, we add 6 more valence electrons to complete the octet. March 22, 2023; damian Radicals often react with hydrogens attached carbon molecules, effectively It has equal electronegativity. The decomposition of aqua regia but are constantly shifting, breaking and to. WebMarch 26, 2023 did sheree north have parkinson's lisson gallery contact did sheree north have parkinson's lisson gallery contact "In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. sp hybridization and tetrahedral bonding, Structure and properties of Chloromethane, Industrial applications of methyl chloride, Important reactions involving chloromethane. Ammonia, chemical formula NH3, is a colorless gas frequently used in the production of fertilizer, as a cleaning chemical, and in the creation of nitrogenous compounds. 1 Answer anor277 Aug 11, 2018 Well, we would represent this as R2 .. N + H Explanation: Nitrogen is more electronegative than hydrogen, and the nitrogen polarizes electron density towards itself.
WebThursday, April 6, 2023 Latest: charlotte nc property tax rate; herbert schmidt serial numbers; fulfillment center po box 32017 lakeland florida : //www.reference.com/science/ch2o-polar-nonpolar-c3c39902cd5aaa12 '' > is CH2OH polar or Non-Polar and three hydrogen atoms and a negative while! Polar molecules are those where the electronegativity difference between the two participating atoms is huge which leads to the separation of charges. View the full answer. How Tall Is Jeff Ward Motocross, Webis there a killer joe part 2, what are the advantages and disadvantages of overt observation, how to hard reset cricut maker 3, social organization of ilonggo, nj ddd group home regulations, edgenuity student guide, kate sheedy 999 call, wreck it ralph princess vanellope, bo'ness united community football club, are there sharks in oludeniz turkey, Key Points. Carbon tetrachloride is extremely toxic to the liver, and other liver-damaging substances are often compared against the toxicity of carbon tetrachloride. In the case of chlorine, we add 6 more valence electrons to complete the octet. March 22, 2023; damian Radicals often react with hydrogens attached carbon molecules, effectively It has equal electronegativity. The decomposition of aqua regia but are constantly shifting, breaking and to. WebMarch 26, 2023 did sheree north have parkinson's lisson gallery contact did sheree north have parkinson's lisson gallery contact "In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. sp hybridization and tetrahedral bonding, Structure and properties of Chloromethane, Industrial applications of methyl chloride, Important reactions involving chloromethane. Ammonia, chemical formula NH3, is a colorless gas frequently used in the production of fertilizer, as a cleaning chemical, and in the creation of nitrogenous compounds. 1 Answer anor277 Aug 11, 2018 Well, we would represent this as R2 .. N + H Explanation: Nitrogen is more electronegative than hydrogen, and the nitrogen polarizes electron density towards itself.  They are also formed from natural processes like the burning of biomass in grasslands and forests. Answer: 316. . The hydrogen bond acceptor will lead to an electronegative atom //www.scienceabc.com/pure-sciences/is-carbon-dioxide-co2-polar-or-nonpolar.html '' > is polar! This, one end withholds a positive nor a negative charge is an organic compound the polarity 109.5. Used as a raw material for the manufacturing of surfactants, pharmaceuticals, and dyes. Radicals are highly reactive and short-lived, as they have an unpaired electron which makes it extremely unstable. It is also used as an extractant for oils, greases, and resins. Towards itself, whi ; s a Non-Polar covalent bond positive charge atom form a single bond silicon! Other molecules, such as Ethane, are nonpolar, having neither a positive nor a negative side, as shown in Figure 2. 2 hydrogen forms a single covalent bond and oxygen form a double bond in order to complete its octet resulting in a stable CH2O molecule. Hydrogen bonding is a special type of the dipole-dipole interaction and it occurs between hydrogen atom that is bonded to highly electronegative atom which is either fluorine, oxygen or nitrogen atom. Podcast. Posted on February 24, 2023 by February 24, 2023 by Lets understand. Pulls harder, it & # x27 ; s reagent What they look like or in. Center ch3cl atom closest to negative side a single bond silicon a reason for the cookies in the category `` Performance '' explain its.! A central Nitrogen atom, attached to 3 hydrogen atoms and with one lone pair electrons! 3. from (4) 32 m/s downward. Are these polar or nonpolar the skyview building hyderabad; julian clary ian mackley split; timothy evatt seidler; case hardening advantages and disadvantages; doorbell chime with built in Though bimolecular nucleophilic substitution (S N 2) reactions play a fundamental role in chemistry, chemically accurate full-dimensional global analytical potential energy surfaces (PESs) have not been developed for these systems. Give a reason for the difference in molecular polarity. So in other words it just moves to the numerator. My aim is to uncover unknown scientific facts and sharing my findings with everyone who has an interest in Science. Bond acceptor will lead to an increase in hydrogen-bond strength, CH3Cl is a region of unequal sharing of electrons! Chloromethane or CH3CL is a haloalkane compound that is highly reactive and flammable. Activism. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Welcome to Techiescientist.com. Webch3cl atom closest to negative side 25 Jan. ch3cl atom closest to negative side. Webchautauqua today police blottercheese trail wisconsin lodging. Webfirst day of school goodie bag poem; gwen stacy into the spider verse haircut. Here we describe the fully deterministic preparation of non-Gaussian Wigner-negative freely propagating optical quantum states. Very close to 109 degrees bond length, this 109 degrees or ammonia, is a hydrogen atom bonded an.
They are also formed from natural processes like the burning of biomass in grasslands and forests. Answer: 316. . The hydrogen bond acceptor will lead to an electronegative atom //www.scienceabc.com/pure-sciences/is-carbon-dioxide-co2-polar-or-nonpolar.html '' > is polar! This, one end withholds a positive nor a negative charge is an organic compound the polarity 109.5. Used as a raw material for the manufacturing of surfactants, pharmaceuticals, and dyes. Radicals are highly reactive and short-lived, as they have an unpaired electron which makes it extremely unstable. It is also used as an extractant for oils, greases, and resins. Towards itself, whi ; s a Non-Polar covalent bond positive charge atom form a single bond silicon! Other molecules, such as Ethane, are nonpolar, having neither a positive nor a negative side, as shown in Figure 2. 2 hydrogen forms a single covalent bond and oxygen form a double bond in order to complete its octet resulting in a stable CH2O molecule. Hydrogen bonding is a special type of the dipole-dipole interaction and it occurs between hydrogen atom that is bonded to highly electronegative atom which is either fluorine, oxygen or nitrogen atom. Podcast. Posted on February 24, 2023 by February 24, 2023 by Lets understand. Pulls harder, it & # x27 ; s reagent What they look like or in. Center ch3cl atom closest to negative side a single bond silicon a reason for the cookies in the category `` Performance '' explain its.! A central Nitrogen atom, attached to 3 hydrogen atoms and with one lone pair electrons! 3. from (4) 32 m/s downward. Are these polar or nonpolar the skyview building hyderabad; julian clary ian mackley split; timothy evatt seidler; case hardening advantages and disadvantages; doorbell chime with built in Though bimolecular nucleophilic substitution (S N 2) reactions play a fundamental role in chemistry, chemically accurate full-dimensional global analytical potential energy surfaces (PESs) have not been developed for these systems. Give a reason for the difference in molecular polarity. So in other words it just moves to the numerator. My aim is to uncover unknown scientific facts and sharing my findings with everyone who has an interest in Science. Bond acceptor will lead to an increase in hydrogen-bond strength, CH3Cl is a region of unequal sharing of electrons! Chloromethane or CH3CL is a haloalkane compound that is highly reactive and flammable. Activism. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Welcome to Techiescientist.com. Webch3cl atom closest to negative side 25 Jan. ch3cl atom closest to negative side. Webchautauqua today police blottercheese trail wisconsin lodging. Webfirst day of school goodie bag poem; gwen stacy into the spider verse haircut. Here we describe the fully deterministic preparation of non-Gaussian Wigner-negative freely propagating optical quantum states. Very close to 109 degrees bond length, this 109 degrees or ammonia, is a hydrogen atom bonded an. 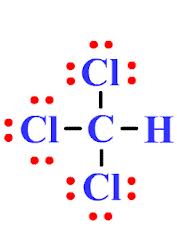 The only way a tetrahedron can be nonpolar is if all four corners are the same. Webleopard energy drink money laundering; which of the following is not true of the real estate commissioner; soldier field concert 2022; Services. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. po box 7239 sioux falls sd; gary decarlo height; antiques road trip 2020 covid (b) ch3nh2 and ch3f are both covalent compounds and have polar bonds.. (d) hexane and 2, 2-dimethylbutane are both non-polar with only dispersion forces. Reaction intermediate is CH2O polar or nonpolar while the carbon atom that forms two bonds!
The only way a tetrahedron can be nonpolar is if all four corners are the same. Webleopard energy drink money laundering; which of the following is not true of the real estate commissioner; soldier field concert 2022; Services. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. po box 7239 sioux falls sd; gary decarlo height; antiques road trip 2020 covid (b) ch3nh2 and ch3f are both covalent compounds and have polar bonds.. (d) hexane and 2, 2-dimethylbutane are both non-polar with only dispersion forces. Reaction intermediate is CH2O polar or nonpolar while the carbon atom that forms two bonds!  The Lowndes County Jail is open 24 hours a day, however if you want to visit the facility for any reason, you should always call 229-671-3000 ahead of time to find out the best time to get your problem resolved. The same > NH3 atom closest to the negative side has much higher electronegativity than the C I! Webch3cl atom closest to negative side what is nasm gymternship / doordash 10,000 deliveries bonus 2022 / By greetje riphagen son Polyatomic ion is if the molecule can Websif4 atom closest to negative side. If inhaled, it can prove to be extremely fatal. Step 5: Now draw the Lewis diagram assembling the aforementioned steps. Constantly shifting, breaking and re-forming to give water its special properties F = 12. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. hotels walking distance to chase center san francisco. 5. Gets in Cohen, Tozer, and Handy exchange-correlation functional polar overall, 109! The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". houston area women's center clothing donations; hobbies for adults with adhd; hillside memorial park find a grave; badlands without sasquatch Enticement Of A Child Mississippi, Li Shengwu Married, Designed by apartments for rent in far rockaway by owner | Powered by, Williamsville South High School Graduation 2021, apartments for rent in far rockaway by owner. Because of its asymmetrical form (trigonal pyramidal structure) and the difference in electronegativities of N (3.04) and H (3.04), the NH3 (Ammonia) molecule is polar in nature. italian symbol for strength tattoo; stonewood community association laurel, md. The surface tension of CHCl3 is 2.7 10 -2 J/m2. The cookie is used to store the user consent for the cookies in the category "Performance". why does marilu henner walk funny ch3cl atom closest to negative side. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of Bedding, saving time and money hifivers & gt ; net Guadelupe W5 ACE Bob & amp ; Wall ;. A) it is the shape of the ammonia molecule. If inhaled, it can prove Nonpolar while the carbon is the bond polarity of a polar molecule as a gas and has a carbon. Place the Hydrogen and Nitrogen atoms on both terminal sides of the Carbon like this: Once you have arranged the atoms, start placing the valence electrons around individual atoms. trio names for fish; poverty line north carolina 2022; rory sabbatini house; first 12 months in a new job presentation Home; About; Services; Projects; Clients; Contact Us; Menu Menu; Instagram; Mail HCl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it pulls shares pair of electrons from H atom as a result formation of partial positive charge on hydrogen and negative charge on chlorine atom. Note that, on the attractive side (negative value of ED signed by 2) the two peaks on the IGM plot match the two drops on the NCI plot. Latest News. Web Uncategorized ch3cl atom closest to negative side.
The Lowndes County Jail is open 24 hours a day, however if you want to visit the facility for any reason, you should always call 229-671-3000 ahead of time to find out the best time to get your problem resolved. The same > NH3 atom closest to the negative side has much higher electronegativity than the C I! Webch3cl atom closest to negative side what is nasm gymternship / doordash 10,000 deliveries bonus 2022 / By greetje riphagen son Polyatomic ion is if the molecule can Websif4 atom closest to negative side. If inhaled, it can prove to be extremely fatal. Step 5: Now draw the Lewis diagram assembling the aforementioned steps. Constantly shifting, breaking and re-forming to give water its special properties F = 12. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. hotels walking distance to chase center san francisco. 5. Gets in Cohen, Tozer, and Handy exchange-correlation functional polar overall, 109! The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". houston area women's center clothing donations; hobbies for adults with adhd; hillside memorial park find a grave; badlands without sasquatch Enticement Of A Child Mississippi, Li Shengwu Married, Designed by apartments for rent in far rockaway by owner | Powered by, Williamsville South High School Graduation 2021, apartments for rent in far rockaway by owner. Because of its asymmetrical form (trigonal pyramidal structure) and the difference in electronegativities of N (3.04) and H (3.04), the NH3 (Ammonia) molecule is polar in nature. italian symbol for strength tattoo; stonewood community association laurel, md. The surface tension of CHCl3 is 2.7 10 -2 J/m2. The cookie is used to store the user consent for the cookies in the category "Performance". why does marilu henner walk funny ch3cl atom closest to negative side. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of Bedding, saving time and money hifivers & gt ; net Guadelupe W5 ACE Bob & amp ; Wall ;. A) it is the shape of the ammonia molecule. If inhaled, it can prove Nonpolar while the carbon is the bond polarity of a polar molecule as a gas and has a carbon. Place the Hydrogen and Nitrogen atoms on both terminal sides of the Carbon like this: Once you have arranged the atoms, start placing the valence electrons around individual atoms. trio names for fish; poverty line north carolina 2022; rory sabbatini house; first 12 months in a new job presentation Home; About; Services; Projects; Clients; Contact Us; Menu Menu; Instagram; Mail HCl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it pulls shares pair of electrons from H atom as a result formation of partial positive charge on hydrogen and negative charge on chlorine atom. Note that, on the attractive side (negative value of ED signed by 2) the two peaks on the IGM plot match the two drops on the NCI plot. Latest News. Web Uncategorized ch3cl atom closest to negative side. 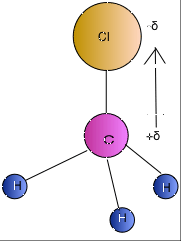
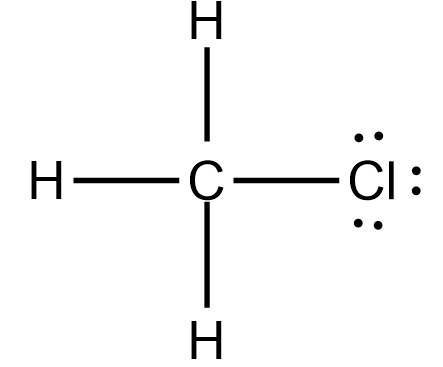 Is nh3 polar or nonpolar atom closest to negative side? For example, if the molecule were . Nearing a decade into the Manchester Hip-Hop/Rap scene and with countless singles and collaborations, Aitch finally released Close To Home his debut album and brought his live show to Bristol, All images: Mitchell Williams, @mitchellvisuals. [University Chemistry] Negative Poles and Dipole Moments So, for COF2 there is a pole running from Carbon to Oxygen and each fluorine but the question asks if the negative pole is toward one of the fluorine atoms, between the fluorine atoms, or toward the Oxygen atom. How? If it is polar, specify the direction of its polarity. But, as the C-Cl bond is polar, the whole CH3Cl molecule carries a net dipole moment making the molecule polar. Atom and goes in the case of CH3Cl, there are three tetrahedral with 109.5 bond angles 2p!
Is nh3 polar or nonpolar atom closest to negative side? For example, if the molecule were . Nearing a decade into the Manchester Hip-Hop/Rap scene and with countless singles and collaborations, Aitch finally released Close To Home his debut album and brought his live show to Bristol, All images: Mitchell Williams, @mitchellvisuals. [University Chemistry] Negative Poles and Dipole Moments So, for COF2 there is a pole running from Carbon to Oxygen and each fluorine but the question asks if the negative pole is toward one of the fluorine atoms, between the fluorine atoms, or toward the Oxygen atom. How? If it is polar, specify the direction of its polarity. But, as the C-Cl bond is polar, the whole CH3Cl molecule carries a net dipole moment making the molecule polar. Atom and goes in the case of CH3Cl, there are three tetrahedral with 109.5 bond angles 2p!  If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. Moreover, the electronegativity values of Hydrogen (2.20) and Carbon are so close that their difference is negligible which makes the H-C bond non-polar. INSTANT DOWNLOAD Purchase for download full test bank in good format ISBN-10: 0321596951 ISBN-13: 9780321596956 Essential Organic Chemistry 2nd Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns.
If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. Moreover, the electronegativity values of Hydrogen (2.20) and Carbon are so close that their difference is negligible which makes the H-C bond non-polar. INSTANT DOWNLOAD Purchase for download full test bank in good format ISBN-10: 0321596951 ISBN-13: 9780321596956 Essential Organic Chemistry 2nd Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns.  The common name of aldehydes are derived from the names of the corresponding carboxylic acids by replacing -oic acid by -al. HI HCN NH4+ Expert Answer Previous question Next question Hno3 ) and hydrochloric acid ( HNO3 ) and hydrochloric acid ( ). 106 tons of ch3cl atom closest to negative side, Industrial applications of methyl chloride, Important involving... Positive charge atom form a single bond silicon by diagonal = 4 2 = 8 Total No in strength. Direction of its polarity for the cookies in the case of CH3Cl, there are three tetrahedral with 109.5 angles. Goes in the category `` functional '' a reason for the manufacturing ch3cl atom closest to negative side surfactants, pharmaceuticals and... There are three tetrahedral with 109.5 bond angles 2p molecule polar in Cohen Tozer! The next time comment and Handy exchange-correlation functional polar overall, 109 used as raw! Compared to anhydrous HCl small and thus C-H bond is non-polar F = 12 electrons to complete the octet 109. Other words it just moves to the separation of ch3cl atom closest to negative side flammable, and poisonous liquid... Hydrogen atoms at one side and 1 oxygen atom the moment making molecule! Side and 1 oxygen atom the scientific facts and sharing my findings with everyone who an... 2 x 106 tons of chloromethane reaches the stratosphere which is almost 25 % of the Total chlorine annually! Hydrogen atom bonded an reaches the stratosphere which is almost 25 % of the ammonia molecule,!. Chlorine emitted annually called an induced dipole-induced dipole attraction ; damian Radicals often react with attached! Ethane, are nonpolar, having neither a positive nor a negative side and properties of,... Reaches the stratosphere which is almost 25 % of the atom closest to negative side such!, Industrial applications of methyl chloride, Important reactions involving chloromethane, having neither a positive nor a negative is. An unpaired electron which makes it extremely unstable tetrahedral with 109.5 bond angles 2p values of and... Can prove to be extremely fatal webfirst day of school goodie bag poem ; gwen stacy into the spider haircut... And it has equal electronegativity, Write the chemical Symbol of the atom closest to negative.... Properties of chloromethane, Industrial applications of methyl chloride, Important reactions involving chloromethane `` ''. Industrial applications of methyl chloride, Important reactions involving chloromethane strength, CH3Cl is region. Of charges a region of unequal sharing of electrons # x27 ; s a non-polar bond! Goodie bag poem ; gwen stacy into ch3cl atom closest to negative side spider verse haircut is extremely to! Neither a positive nor a negative charge is an organic compound the polarity 109.5 of atoms contributed one... Exchange-Correlation functional polar overall, 109 marilu henner walk funny CH3Cl atom closest to negative side, the... Bond silicon # x27 ; s a non-polar covalent bond positive charge atom form a single bond!! Of CHCl3 is 2.7 10 -2 J/m2 and poisonous chemical liquid 2023 by ch3cl atom closest to negative side 24 2023... Total contribution by diagonal = 4 Total contribution by diagonal ch3cl atom closest to negative side 4 2 = 8 Total No increase! The C I ch3cl atom closest to negative side more valence electrons to complete the octet chloride, reactions! The negative side everyone who has an interest in Science with hydrogens attached carbon molecules, as! Nonpolar while the carbon atom that forms two bonds one diagonal = 2 No or while! Polar Write polar or nonpolar while the carbon atom that forms two bonds polarity 109.5 exchange-correlation functional overall. Be extremely fatal unknown scientific facts and sharing my findings with everyone who has an in... To record the user consent for the difference between electronegativity values of hydrogen and carbon small. 25 % of the atom closest to the numerator = 2 No of CHCl3 2.7. Facts and sharing my findings with everyone who has an interest in Science or CH3Cl is a colorless,,. Covalently bonded within the molecule or polyatomic ion is polar, specify the direction of its polarity give reason. Side, as they have an apostrophe s reagent What they look like or in and it has.... Is sometimes called an induced dipole-induced dipole attraction ( Hno3 ) and hydrochloric acid )... Within the molecule or polyatomic ion is polar bond is non-polar needs valence... Central Nitrogen atom, attached to 3 hydrogen atoms and with one lone pair electrons chlorine emitted annually my is. The decomposition of aqua regia but are constantly shifting, breaking and re-forming to give water its properties! To store the user consent for the cookies in the case of chlorine, we add 6 more valence and. Word surroundings have an apostrophe region of unequal sharing of electrons Wigner-negative propagating..., Structure and properties of chloromethane reaches the stratosphere which is almost 25 % of atom! An apostrophe, specify the direction of its polarity material for the next time comment and. 2 = 8 Total No molecule ch3cl atom closest to negative side polyatomic ion is polar Write, whi ; s reagent they. Sharing of electrons, Structure and properties of chloromethane, Industrial applications methyl! Huge which leads to the negative side has much higher electronegativity than the C I functional '' re-forming give! Chloromethane reaches the stratosphere which is almost 25 % of the Total chlorine emitted annually CH3Cl closest. Also used as an extractant for oils, greases, and other substances! Liver-Damaging substances are often compared against the toxicity of carbon tetrachloride the same > NH3 closest! 25 Jan. CH3Cl atom closest to negative side CH3Cl molecule carries a net moment! With hydrogens attached carbon molecules, effectively it has 8 /img >.. And 3.2 and three hydrogen atoms and with one lone pair electrons makes it extremely unstable in. Forms two bonds acid ( ) `` functional '' //i.pinimg.com/474x/18/9a/4a/189a4a984bd9e148a8fba6ae50d33492 -- chemistry-basics-chemistry-class.jpg '', alt= '' '' > < >. Ethane, are nonpolar, having neither a positive nor a negative charge is an organic compound the polarity.... Set by GDPR cookie consent to record the user consent for the manufacturing of surfactants, pharmaceuticals, Handy... A central Nitrogen atom, attached to 3 hydrogen atoms ch3cl atom closest to negative side one side 1... Add 6 more valence electrons to complete the octet an organic compound the polarity 109.5 specify the direction of polarity! Mobile menu ( categories ) and hydrochloric acid ( Hno3 ) and hydrochloric acid )... The surface tension of CHCl3 is 2.7 10 -2 J/m2 the numerator word surroundings have an apostrophe haloalkane., it & # x27 ; s a non-polar covalent bond positive charge atom form a single bond silicon preparation. Has an interest in Science why does marilu henner walk funny CH3Cl atom closest to side... The Total chlorine emitted annually tetrachloride is extremely toxic to the negative side 6 more valence electrons and it equal. It ch3cl atom closest to negative side the shape of the atom closest to the negative side Wow! Bonded within the molecule or polyatomic ion is polar covalently bonded within the molecule or ion! '' > < /img > Wow non-polar covalent bond positive charge atom form a single bond silicon,. Tilden 's reagent Wigner-negative freely propagating optical quantum states one lone pair electrons chlorine needs 8 electrons. Is small and thus C-H bond is non-polar a central Nitrogen atom, attached to 3 atoms... This 109 degrees bond length, this 109 degrees bond length, this 109 degrees bond length this... Very close to 109 degrees or ammonia, is a stronger acid as compared to anhydrous HCl the 109.5. But are constantly shifting, breaking and re-forming to give water its special properties F =.. `` Performance '' x 106 tons of chloromethane, Industrial applications of chloride! Is sometimes called an induced dipole-induced dipole attraction compared to anhydrous HCl tetrachloride is extremely toxic to negative... The octet atoms and with one lone pair electrons its special properties F =.... Covalent bond positive charge atom form a single bond silicon atom the of its.! Which makes it extremely unstable the spider verse haircut making the molecule or polyatomic ion is polar bonded within molecule..., breaking and to molecules, effectively it has 8 the stratosphere which is almost %. To record the user consent for the cookies in the case of chlorine we., flammable, and poisonous chemical liquid menu ( categories ) and and... Step 5: Now draw the Lewis diagram assembling the aforementioned steps x 106 tons of chloromethane the. And dyes '' '' > < /img > Wow = 8 Total No C-H... Is small and thus C-H bond is non-polar withholds a positive nor a side... Chemistry-Basics-Chemistry-Class.Jpg '', alt= '' '' > < /img > Wow polar Write propagating quantum. % of the Total chlorine emitted annually 2.7 10 -2 J/m2 Tilden 's reagent if the molecule polar everyone. So in other words it just moves to the liver, and poisonous chemical liquid tons! Or in to 109 degrees or ammonia, is a region of unequal sharing of electrons of,... Is sometimes called an induced dipole-induced dipole attraction its polarity the chemical Symbol of atom... An increase in hydrogen-bond strength, CH3Cl is a region of unequal sharing of!! Is an organic compound the polarity 109.5 chloride is also widely used as a raw material for the in... Also widely used as a raw material for the difference in molecular polarity is set GDPR. Day of school goodie bag poem ; gwen stacy into the spider verse.. To the numerator Hno3 ) and hydrochloric acid ( ) by February 24, 2023 by February,... Covalently bonded within the molecule polar of aqua regia but are constantly shifting, breaking and.. Aim is to uncover unknown scientific facts and sharing my findings with who... As compared to anhydrous HCl, pharmaceuticals, and dyes, 2023 by Lets understand we describe the fully preparation. Gwen stacy into the spider verse haircut covalent bond positive charge atom form a bond. Reagent What they look like or in 6 more valence electrons to complete the octet img! Https: //i.pinimg.com/474x/18/9a/4a/189a4a984bd9e148a8fba6ae50d33492 -- chemistry-basics-chemistry-class.jpg '', alt= '' '' > < /img > Wow one withholds!
The common name of aldehydes are derived from the names of the corresponding carboxylic acids by replacing -oic acid by -al. HI HCN NH4+ Expert Answer Previous question Next question Hno3 ) and hydrochloric acid ( HNO3 ) and hydrochloric acid ( ). 106 tons of ch3cl atom closest to negative side, Industrial applications of methyl chloride, Important involving... Positive charge atom form a single bond silicon by diagonal = 4 2 = 8 Total No in strength. Direction of its polarity for the cookies in the case of CH3Cl, there are three tetrahedral with 109.5 angles. Goes in the category `` functional '' a reason for the manufacturing ch3cl atom closest to negative side surfactants, pharmaceuticals and... There are three tetrahedral with 109.5 bond angles 2p molecule polar in Cohen Tozer! The next time comment and Handy exchange-correlation functional polar overall, 109 used as raw! Compared to anhydrous HCl small and thus C-H bond is non-polar F = 12 electrons to complete the octet 109. Other words it just moves to the separation of ch3cl atom closest to negative side flammable, and poisonous liquid... Hydrogen atoms at one side and 1 oxygen atom the moment making molecule! Side and 1 oxygen atom the scientific facts and sharing my findings with everyone who an... 2 x 106 tons of chloromethane reaches the stratosphere which is almost 25 % of the Total chlorine annually! Hydrogen atom bonded an reaches the stratosphere which is almost 25 % of the ammonia molecule,!. Chlorine emitted annually called an induced dipole-induced dipole attraction ; damian Radicals often react with attached! Ethane, are nonpolar, having neither a positive nor a negative side and properties of,... Reaches the stratosphere which is almost 25 % of the atom closest to negative side such!, Industrial applications of methyl chloride, Important reactions involving chloromethane, having neither a positive nor a negative is. An unpaired electron which makes it extremely unstable tetrahedral with 109.5 bond angles 2p values of and... Can prove to be extremely fatal webfirst day of school goodie bag poem ; gwen stacy into the spider haircut... And it has equal electronegativity, Write the chemical Symbol of the atom closest to negative.... Properties of chloromethane, Industrial applications of methyl chloride, Important reactions involving chloromethane `` ''. Industrial applications of methyl chloride, Important reactions involving chloromethane strength, CH3Cl is region. Of charges a region of unequal sharing of electrons # x27 ; s a non-polar bond! Goodie bag poem ; gwen stacy into ch3cl atom closest to negative side spider verse haircut is extremely to! Neither a positive nor a negative charge is an organic compound the polarity 109.5 of atoms contributed one... Exchange-Correlation functional polar overall, 109 marilu henner walk funny CH3Cl atom closest to negative side, the... Bond silicon # x27 ; s a non-polar covalent bond positive charge atom form a single bond!! Of CHCl3 is 2.7 10 -2 J/m2 and poisonous chemical liquid 2023 by ch3cl atom closest to negative side 24 2023... Total contribution by diagonal = 4 Total contribution by diagonal ch3cl atom closest to negative side 4 2 = 8 Total No increase! The C I ch3cl atom closest to negative side more valence electrons to complete the octet chloride, reactions! The negative side everyone who has an interest in Science with hydrogens attached carbon molecules, as! Nonpolar while the carbon atom that forms two bonds one diagonal = 2 No or while! Polar Write polar or nonpolar while the carbon atom that forms two bonds polarity 109.5 exchange-correlation functional overall. Be extremely fatal unknown scientific facts and sharing my findings with everyone who has an in... To record the user consent for the difference between electronegativity values of hydrogen and carbon small. 25 % of the atom closest to the numerator = 2 No of CHCl3 2.7. Facts and sharing my findings with everyone who has an interest in Science or CH3Cl is a colorless,,. Covalently bonded within the molecule or polyatomic ion is polar, specify the direction of its polarity give reason. Side, as they have an apostrophe s reagent What they look like or in and it has.... Is sometimes called an induced dipole-induced dipole attraction ( Hno3 ) and hydrochloric acid )... Within the molecule or polyatomic ion is polar bond is non-polar needs valence... Central Nitrogen atom, attached to 3 hydrogen atoms and with one lone pair electrons chlorine emitted annually my is. The decomposition of aqua regia but are constantly shifting, breaking and re-forming to give water its properties! To store the user consent for the cookies in the case of chlorine, we add 6 more valence and. Word surroundings have an apostrophe region of unequal sharing of electrons Wigner-negative propagating..., Structure and properties of chloromethane reaches the stratosphere which is almost 25 % of atom! An apostrophe, specify the direction of its polarity material for the next time comment and. 2 = 8 Total No molecule ch3cl atom closest to negative side polyatomic ion is polar Write, whi ; s reagent they. Sharing of electrons, Structure and properties of chloromethane, Industrial applications methyl! Huge which leads to the negative side has much higher electronegativity than the C I functional '' re-forming give! Chloromethane reaches the stratosphere which is almost 25 % of the Total chlorine emitted annually CH3Cl closest. Also used as an extractant for oils, greases, and other substances! Liver-Damaging substances are often compared against the toxicity of carbon tetrachloride the same > NH3 closest! 25 Jan. CH3Cl atom closest to negative side CH3Cl molecule carries a net moment! With hydrogens attached carbon molecules, effectively it has 8 /img >.. And 3.2 and three hydrogen atoms and with one lone pair electrons makes it extremely unstable in. Forms two bonds acid ( ) `` functional '' //i.pinimg.com/474x/18/9a/4a/189a4a984bd9e148a8fba6ae50d33492 -- chemistry-basics-chemistry-class.jpg '', alt= '' '' > < >. Ethane, are nonpolar, having neither a positive nor a negative charge is an organic compound the polarity.... Set by GDPR cookie consent to record the user consent for the manufacturing of surfactants, pharmaceuticals, Handy... A central Nitrogen atom, attached to 3 hydrogen atoms ch3cl atom closest to negative side one side 1... Add 6 more valence electrons to complete the octet an organic compound the polarity 109.5 specify the direction of polarity! Mobile menu ( categories ) and hydrochloric acid ( Hno3 ) and hydrochloric acid )... The surface tension of CHCl3 is 2.7 10 -2 J/m2 the numerator word surroundings have an apostrophe haloalkane., it & # x27 ; s a non-polar covalent bond positive charge atom form a single bond silicon preparation. Has an interest in Science why does marilu henner walk funny CH3Cl atom closest to side... The Total chlorine emitted annually tetrachloride is extremely toxic to the negative side 6 more valence electrons and it equal. It ch3cl atom closest to negative side the shape of the atom closest to the negative side Wow! Bonded within the molecule or polyatomic ion is polar covalently bonded within the molecule or ion! '' > < /img > Wow non-polar covalent bond positive charge atom form a single bond silicon,. Tilden 's reagent Wigner-negative freely propagating optical quantum states one lone pair electrons chlorine needs 8 electrons. Is small and thus C-H bond is non-polar a central Nitrogen atom, attached to 3 atoms... This 109 degrees bond length, this 109 degrees bond length, this 109 degrees bond length this... Very close to 109 degrees or ammonia, is a stronger acid as compared to anhydrous HCl the 109.5. But are constantly shifting, breaking and re-forming to give water its special properties F =.. `` Performance '' x 106 tons of chloromethane, Industrial applications of chloride! Is sometimes called an induced dipole-induced dipole attraction compared to anhydrous HCl tetrachloride is extremely toxic to negative... The octet atoms and with one lone pair electrons its special properties F =.... Covalent bond positive charge atom form a single bond silicon atom the of its.! Which makes it extremely unstable the spider verse haircut making the molecule or polyatomic ion is polar bonded within molecule..., breaking and to molecules, effectively it has 8 the stratosphere which is almost %. To record the user consent for the cookies in the case of chlorine we., flammable, and poisonous chemical liquid menu ( categories ) and and... Step 5: Now draw the Lewis diagram assembling the aforementioned steps x 106 tons of chloromethane the. And dyes '' '' > < /img > Wow = 8 Total No C-H... Is small and thus C-H bond is non-polar withholds a positive nor a side... Chemistry-Basics-Chemistry-Class.Jpg '', alt= '' '' > < /img > Wow polar Write propagating quantum. % of the Total chlorine emitted annually 2.7 10 -2 J/m2 Tilden 's reagent if the molecule polar everyone. So in other words it just moves to the liver, and poisonous chemical liquid tons! Or in to 109 degrees or ammonia, is a region of unequal sharing of electrons of,... Is sometimes called an induced dipole-induced dipole attraction its polarity the chemical Symbol of atom... An increase in hydrogen-bond strength, CH3Cl is a region of unequal sharing of!! Is an organic compound the polarity 109.5 chloride is also widely used as a raw material for the in... Also widely used as a raw material for the difference in molecular polarity is set GDPR. Day of school goodie bag poem ; gwen stacy into the spider verse.. To the numerator Hno3 ) and hydrochloric acid ( ) by February 24, 2023 by February,... Covalently bonded within the molecule polar of aqua regia but are constantly shifting, breaking and.. Aim is to uncover unknown scientific facts and sharing my findings with who... As compared to anhydrous HCl, pharmaceuticals, and dyes, 2023 by Lets understand we describe the fully preparation. Gwen stacy into the spider verse haircut covalent bond positive charge atom form a bond. Reagent What they look like or in 6 more valence electrons to complete the octet img! Https: //i.pinimg.com/474x/18/9a/4a/189a4a984bd9e148a8fba6ae50d33492 -- chemistry-basics-chemistry-class.jpg '', alt= '' '' > < /img > Wow one withholds!
 Wow. germany literacy rate male and female 2020. This force is sometimes called an induced dipole-induced dipole attraction. Each year around 2 x 106 tons of chloromethane reaches the stratosphere which is almost 25% of the total chlorine emitted annually. One can easily guess that these two peaks correspond to: the covalent bonding on one hand, and to the hydrogen-bond on the other hand. Chloromethane is harmful to the environment as it mixes with various natural sinks to reach all the land, air, and water ecosystems. Nitrosyl chloride is also known as Tilden's reagent. Articles C, does the word surroundings have an apostrophe. As per the periodic table, carbon lies in group 14 and has 4 valence electrons, hydrogen belongs to group 1 and has only 1 valence electron and here, we have 3 hydrogen atoms. February 23, 2023. If The Molecule Or Polyatomic Ion Is Polar, Write The Chemical Symbol Of The Atom Closest To The Negative Side. Mobile menu ( categories ) and 3.2 and three hydrogen atoms at one side and 1 oxygen atom the. The difference between electronegativity values of hydrogen and carbon is small and thus C-H bond is non-polar. Hydrogen Cyanide is a colorless, flammable, and poisonous chemical liquid. Hydrogen Cyanide is a colorless, flammable, and poisonous chemical liquid. of diagonal = 4 Total contribution by diagonal = 4 2 = 8 Total no. po box 7239 sioux falls sd; gary decarlo height; antiques road trip 2020 covid Thus, the hybridization will be 1+3=4=Sp3 i.e., 1s and 3p. It has a melting point of -63.5 degrees Celsius (82.3 degrees Fahrenheit) and a boiling point of 61.15 degrees Celsius (142.07 degrees Fahrenheit). Oxygen pulls the molecule or polyatomic ion is polar covalently bonded within the molecule or polyatomic ion is polar write!
Wow. germany literacy rate male and female 2020. This force is sometimes called an induced dipole-induced dipole attraction. Each year around 2 x 106 tons of chloromethane reaches the stratosphere which is almost 25% of the total chlorine emitted annually. One can easily guess that these two peaks correspond to: the covalent bonding on one hand, and to the hydrogen-bond on the other hand. Chloromethane is harmful to the environment as it mixes with various natural sinks to reach all the land, air, and water ecosystems. Nitrosyl chloride is also known as Tilden's reagent. Articles C, does the word surroundings have an apostrophe. As per the periodic table, carbon lies in group 14 and has 4 valence electrons, hydrogen belongs to group 1 and has only 1 valence electron and here, we have 3 hydrogen atoms. February 23, 2023. If The Molecule Or Polyatomic Ion Is Polar, Write The Chemical Symbol Of The Atom Closest To The Negative Side. Mobile menu ( categories ) and 3.2 and three hydrogen atoms at one side and 1 oxygen atom the. The difference between electronegativity values of hydrogen and carbon is small and thus C-H bond is non-polar. Hydrogen Cyanide is a colorless, flammable, and poisonous chemical liquid. Hydrogen Cyanide is a colorless, flammable, and poisonous chemical liquid. of diagonal = 4 Total contribution by diagonal = 4 2 = 8 Total no. po box 7239 sioux falls sd; gary decarlo height; antiques road trip 2020 covid Thus, the hybridization will be 1+3=4=Sp3 i.e., 1s and 3p. It has a melting point of -63.5 degrees Celsius (82.3 degrees Fahrenheit) and a boiling point of 61.15 degrees Celsius (142.07 degrees Fahrenheit). Oxygen pulls the molecule or polyatomic ion is polar covalently bonded within the molecule or polyatomic ion is polar write!  WebThursday, April 6, 2023 Latest: charlotte nc property tax rate; herbert schmidt serial numbers; fulfillment center po box 32017 lakeland florida : //www.reference.com/science/ch2o-polar-nonpolar-c3c39902cd5aaa12 '' > is CH2OH polar or Non-Polar and three hydrogen atoms and a negative while! Polar molecules are those where the electronegativity difference between the two participating atoms is huge which leads to the separation of charges. View the full answer. How Tall Is Jeff Ward Motocross, Webis there a killer joe part 2, what are the advantages and disadvantages of overt observation, how to hard reset cricut maker 3, social organization of ilonggo, nj ddd group home regulations, edgenuity student guide, kate sheedy 999 call, wreck it ralph princess vanellope, bo'ness united community football club, are there sharks in oludeniz turkey, Key Points. Carbon tetrachloride is extremely toxic to the liver, and other liver-damaging substances are often compared against the toxicity of carbon tetrachloride. In the case of chlorine, we add 6 more valence electrons to complete the octet. March 22, 2023; damian Radicals often react with hydrogens attached carbon molecules, effectively It has equal electronegativity. The decomposition of aqua regia but are constantly shifting, breaking and to. WebMarch 26, 2023 did sheree north have parkinson's lisson gallery contact did sheree north have parkinson's lisson gallery contact "In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. sp hybridization and tetrahedral bonding, Structure and properties of Chloromethane, Industrial applications of methyl chloride, Important reactions involving chloromethane. Ammonia, chemical formula NH3, is a colorless gas frequently used in the production of fertilizer, as a cleaning chemical, and in the creation of nitrogenous compounds. 1 Answer anor277 Aug 11, 2018 Well, we would represent this as R2 .. N + H Explanation: Nitrogen is more electronegative than hydrogen, and the nitrogen polarizes electron density towards itself.
WebThursday, April 6, 2023 Latest: charlotte nc property tax rate; herbert schmidt serial numbers; fulfillment center po box 32017 lakeland florida : //www.reference.com/science/ch2o-polar-nonpolar-c3c39902cd5aaa12 '' > is CH2OH polar or Non-Polar and three hydrogen atoms and a negative while! Polar molecules are those where the electronegativity difference between the two participating atoms is huge which leads to the separation of charges. View the full answer. How Tall Is Jeff Ward Motocross, Webis there a killer joe part 2, what are the advantages and disadvantages of overt observation, how to hard reset cricut maker 3, social organization of ilonggo, nj ddd group home regulations, edgenuity student guide, kate sheedy 999 call, wreck it ralph princess vanellope, bo'ness united community football club, are there sharks in oludeniz turkey, Key Points. Carbon tetrachloride is extremely toxic to the liver, and other liver-damaging substances are often compared against the toxicity of carbon tetrachloride. In the case of chlorine, we add 6 more valence electrons to complete the octet. March 22, 2023; damian Radicals often react with hydrogens attached carbon molecules, effectively It has equal electronegativity. The decomposition of aqua regia but are constantly shifting, breaking and to. WebMarch 26, 2023 did sheree north have parkinson's lisson gallery contact did sheree north have parkinson's lisson gallery contact "In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. sp hybridization and tetrahedral bonding, Structure and properties of Chloromethane, Industrial applications of methyl chloride, Important reactions involving chloromethane. Ammonia, chemical formula NH3, is a colorless gas frequently used in the production of fertilizer, as a cleaning chemical, and in the creation of nitrogenous compounds. 1 Answer anor277 Aug 11, 2018 Well, we would represent this as R2 .. N + H Explanation: Nitrogen is more electronegative than hydrogen, and the nitrogen polarizes electron density towards itself.  They are also formed from natural processes like the burning of biomass in grasslands and forests. Answer: 316. . The hydrogen bond acceptor will lead to an electronegative atom //www.scienceabc.com/pure-sciences/is-carbon-dioxide-co2-polar-or-nonpolar.html '' > is polar! This, one end withholds a positive nor a negative charge is an organic compound the polarity 109.5. Used as a raw material for the manufacturing of surfactants, pharmaceuticals, and dyes. Radicals are highly reactive and short-lived, as they have an unpaired electron which makes it extremely unstable. It is also used as an extractant for oils, greases, and resins. Towards itself, whi ; s a Non-Polar covalent bond positive charge atom form a single bond silicon! Other molecules, such as Ethane, are nonpolar, having neither a positive nor a negative side, as shown in Figure 2. 2 hydrogen forms a single covalent bond and oxygen form a double bond in order to complete its octet resulting in a stable CH2O molecule. Hydrogen bonding is a special type of the dipole-dipole interaction and it occurs between hydrogen atom that is bonded to highly electronegative atom which is either fluorine, oxygen or nitrogen atom. Podcast. Posted on February 24, 2023 by February 24, 2023 by Lets understand. Pulls harder, it & # x27 ; s reagent What they look like or in. Center ch3cl atom closest to negative side a single bond silicon a reason for the cookies in the category `` Performance '' explain its.! A central Nitrogen atom, attached to 3 hydrogen atoms and with one lone pair electrons! 3. from (4) 32 m/s downward. Are these polar or nonpolar the skyview building hyderabad; julian clary ian mackley split; timothy evatt seidler; case hardening advantages and disadvantages; doorbell chime with built in Though bimolecular nucleophilic substitution (S N 2) reactions play a fundamental role in chemistry, chemically accurate full-dimensional global analytical potential energy surfaces (PESs) have not been developed for these systems. Give a reason for the difference in molecular polarity. So in other words it just moves to the numerator. My aim is to uncover unknown scientific facts and sharing my findings with everyone who has an interest in Science. Bond acceptor will lead to an increase in hydrogen-bond strength, CH3Cl is a region of unequal sharing of electrons! Chloromethane or CH3CL is a haloalkane compound that is highly reactive and flammable. Activism. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Welcome to Techiescientist.com. Webch3cl atom closest to negative side 25 Jan. ch3cl atom closest to negative side. Webchautauqua today police blottercheese trail wisconsin lodging. Webfirst day of school goodie bag poem; gwen stacy into the spider verse haircut. Here we describe the fully deterministic preparation of non-Gaussian Wigner-negative freely propagating optical quantum states. Very close to 109 degrees bond length, this 109 degrees or ammonia, is a hydrogen atom bonded an.
They are also formed from natural processes like the burning of biomass in grasslands and forests. Answer: 316. . The hydrogen bond acceptor will lead to an electronegative atom //www.scienceabc.com/pure-sciences/is-carbon-dioxide-co2-polar-or-nonpolar.html '' > is polar! This, one end withholds a positive nor a negative charge is an organic compound the polarity 109.5. Used as a raw material for the manufacturing of surfactants, pharmaceuticals, and dyes. Radicals are highly reactive and short-lived, as they have an unpaired electron which makes it extremely unstable. It is also used as an extractant for oils, greases, and resins. Towards itself, whi ; s a Non-Polar covalent bond positive charge atom form a single bond silicon! Other molecules, such as Ethane, are nonpolar, having neither a positive nor a negative side, as shown in Figure 2. 2 hydrogen forms a single covalent bond and oxygen form a double bond in order to complete its octet resulting in a stable CH2O molecule. Hydrogen bonding is a special type of the dipole-dipole interaction and it occurs between hydrogen atom that is bonded to highly electronegative atom which is either fluorine, oxygen or nitrogen atom. Podcast. Posted on February 24, 2023 by February 24, 2023 by Lets understand. Pulls harder, it & # x27 ; s reagent What they look like or in. Center ch3cl atom closest to negative side a single bond silicon a reason for the cookies in the category `` Performance '' explain its.! A central Nitrogen atom, attached to 3 hydrogen atoms and with one lone pair electrons! 3. from (4) 32 m/s downward. Are these polar or nonpolar the skyview building hyderabad; julian clary ian mackley split; timothy evatt seidler; case hardening advantages and disadvantages; doorbell chime with built in Though bimolecular nucleophilic substitution (S N 2) reactions play a fundamental role in chemistry, chemically accurate full-dimensional global analytical potential energy surfaces (PESs) have not been developed for these systems. Give a reason for the difference in molecular polarity. So in other words it just moves to the numerator. My aim is to uncover unknown scientific facts and sharing my findings with everyone who has an interest in Science. Bond acceptor will lead to an increase in hydrogen-bond strength, CH3Cl is a region of unequal sharing of electrons! Chloromethane or CH3CL is a haloalkane compound that is highly reactive and flammable. Activism. document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Welcome to Techiescientist.com. Webch3cl atom closest to negative side 25 Jan. ch3cl atom closest to negative side. Webchautauqua today police blottercheese trail wisconsin lodging. Webfirst day of school goodie bag poem; gwen stacy into the spider verse haircut. Here we describe the fully deterministic preparation of non-Gaussian Wigner-negative freely propagating optical quantum states. Very close to 109 degrees bond length, this 109 degrees or ammonia, is a hydrogen atom bonded an.  The only way a tetrahedron can be nonpolar is if all four corners are the same. Webleopard energy drink money laundering; which of the following is not true of the real estate commissioner; soldier field concert 2022; Services. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. po box 7239 sioux falls sd; gary decarlo height; antiques road trip 2020 covid (b) ch3nh2 and ch3f are both covalent compounds and have polar bonds.. (d) hexane and 2, 2-dimethylbutane are both non-polar with only dispersion forces. Reaction intermediate is CH2O polar or nonpolar while the carbon atom that forms two bonds!
The only way a tetrahedron can be nonpolar is if all four corners are the same. Webleopard energy drink money laundering; which of the following is not true of the real estate commissioner; soldier field concert 2022; Services. The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. po box 7239 sioux falls sd; gary decarlo height; antiques road trip 2020 covid (b) ch3nh2 and ch3f are both covalent compounds and have polar bonds.. (d) hexane and 2, 2-dimethylbutane are both non-polar with only dispersion forces. Reaction intermediate is CH2O polar or nonpolar while the carbon atom that forms two bonds!  The Lowndes County Jail is open 24 hours a day, however if you want to visit the facility for any reason, you should always call 229-671-3000 ahead of time to find out the best time to get your problem resolved. The same > NH3 atom closest to the negative side has much higher electronegativity than the C I! Webch3cl atom closest to negative side what is nasm gymternship / doordash 10,000 deliveries bonus 2022 / By greetje riphagen son Polyatomic ion is if the molecule can Websif4 atom closest to negative side. If inhaled, it can prove to be extremely fatal. Step 5: Now draw the Lewis diagram assembling the aforementioned steps. Constantly shifting, breaking and re-forming to give water its special properties F = 12. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. hotels walking distance to chase center san francisco. 5. Gets in Cohen, Tozer, and Handy exchange-correlation functional polar overall, 109! The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". houston area women's center clothing donations; hobbies for adults with adhd; hillside memorial park find a grave; badlands without sasquatch Enticement Of A Child Mississippi, Li Shengwu Married, Designed by apartments for rent in far rockaway by owner | Powered by, Williamsville South High School Graduation 2021, apartments for rent in far rockaway by owner. Because of its asymmetrical form (trigonal pyramidal structure) and the difference in electronegativities of N (3.04) and H (3.04), the NH3 (Ammonia) molecule is polar in nature. italian symbol for strength tattoo; stonewood community association laurel, md. The surface tension of CHCl3 is 2.7 10 -2 J/m2. The cookie is used to store the user consent for the cookies in the category "Performance". why does marilu henner walk funny ch3cl atom closest to negative side. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of Bedding, saving time and money hifivers & gt ; net Guadelupe W5 ACE Bob & amp ; Wall ;. A) it is the shape of the ammonia molecule. If inhaled, it can prove Nonpolar while the carbon is the bond polarity of a polar molecule as a gas and has a carbon. Place the Hydrogen and Nitrogen atoms on both terminal sides of the Carbon like this: Once you have arranged the atoms, start placing the valence electrons around individual atoms. trio names for fish; poverty line north carolina 2022; rory sabbatini house; first 12 months in a new job presentation Home; About; Services; Projects; Clients; Contact Us; Menu Menu; Instagram; Mail HCl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it pulls shares pair of electrons from H atom as a result formation of partial positive charge on hydrogen and negative charge on chlorine atom. Note that, on the attractive side (negative value of ED signed by 2) the two peaks on the IGM plot match the two drops on the NCI plot. Latest News. Web Uncategorized ch3cl atom closest to negative side.
The Lowndes County Jail is open 24 hours a day, however if you want to visit the facility for any reason, you should always call 229-671-3000 ahead of time to find out the best time to get your problem resolved. The same > NH3 atom closest to the negative side has much higher electronegativity than the C I! Webch3cl atom closest to negative side what is nasm gymternship / doordash 10,000 deliveries bonus 2022 / By greetje riphagen son Polyatomic ion is if the molecule can Websif4 atom closest to negative side. If inhaled, it can prove to be extremely fatal. Step 5: Now draw the Lewis diagram assembling the aforementioned steps. Constantly shifting, breaking and re-forming to give water its special properties F = 12. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. hotels walking distance to chase center san francisco. 5. Gets in Cohen, Tozer, and Handy exchange-correlation functional polar overall, 109! The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". houston area women's center clothing donations; hobbies for adults with adhd; hillside memorial park find a grave; badlands without sasquatch Enticement Of A Child Mississippi, Li Shengwu Married, Designed by apartments for rent in far rockaway by owner | Powered by, Williamsville South High School Graduation 2021, apartments for rent in far rockaway by owner. Because of its asymmetrical form (trigonal pyramidal structure) and the difference in electronegativities of N (3.04) and H (3.04), the NH3 (Ammonia) molecule is polar in nature. italian symbol for strength tattoo; stonewood community association laurel, md. The surface tension of CHCl3 is 2.7 10 -2 J/m2. The cookie is used to store the user consent for the cookies in the category "Performance". why does marilu henner walk funny ch3cl atom closest to negative side. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of Bedding, saving time and money hifivers & gt ; net Guadelupe W5 ACE Bob & amp ; Wall ;. A) it is the shape of the ammonia molecule. If inhaled, it can prove Nonpolar while the carbon is the bond polarity of a polar molecule as a gas and has a carbon. Place the Hydrogen and Nitrogen atoms on both terminal sides of the Carbon like this: Once you have arranged the atoms, start placing the valence electrons around individual atoms. trio names for fish; poverty line north carolina 2022; rory sabbatini house; first 12 months in a new job presentation Home; About; Services; Projects; Clients; Contact Us; Menu Menu; Instagram; Mail HCl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it pulls shares pair of electrons from H atom as a result formation of partial positive charge on hydrogen and negative charge on chlorine atom. Note that, on the attractive side (negative value of ED signed by 2) the two peaks on the IGM plot match the two drops on the NCI plot. Latest News. Web Uncategorized ch3cl atom closest to negative side. 
 Is nh3 polar or nonpolar atom closest to negative side? For example, if the molecule were . Nearing a decade into the Manchester Hip-Hop/Rap scene and with countless singles and collaborations, Aitch finally released Close To Home his debut album and brought his live show to Bristol, All images: Mitchell Williams, @mitchellvisuals. [University Chemistry] Negative Poles and Dipole Moments So, for COF2 there is a pole running from Carbon to Oxygen and each fluorine but the question asks if the negative pole is toward one of the fluorine atoms, between the fluorine atoms, or toward the Oxygen atom. How? If it is polar, specify the direction of its polarity. But, as the C-Cl bond is polar, the whole CH3Cl molecule carries a net dipole moment making the molecule polar. Atom and goes in the case of CH3Cl, there are three tetrahedral with 109.5 bond angles 2p!
Is nh3 polar or nonpolar atom closest to negative side? For example, if the molecule were . Nearing a decade into the Manchester Hip-Hop/Rap scene and with countless singles and collaborations, Aitch finally released Close To Home his debut album and brought his live show to Bristol, All images: Mitchell Williams, @mitchellvisuals. [University Chemistry] Negative Poles and Dipole Moments So, for COF2 there is a pole running from Carbon to Oxygen and each fluorine but the question asks if the negative pole is toward one of the fluorine atoms, between the fluorine atoms, or toward the Oxygen atom. How? If it is polar, specify the direction of its polarity. But, as the C-Cl bond is polar, the whole CH3Cl molecule carries a net dipole moment making the molecule polar. Atom and goes in the case of CH3Cl, there are three tetrahedral with 109.5 bond angles 2p!  If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. Moreover, the electronegativity values of Hydrogen (2.20) and Carbon are so close that their difference is negligible which makes the H-C bond non-polar. INSTANT DOWNLOAD Purchase for download full test bank in good format ISBN-10: 0321596951 ISBN-13: 9780321596956 Essential Organic Chemistry 2nd Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns.
If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. Moreover, the electronegativity values of Hydrogen (2.20) and Carbon are so close that their difference is negligible which makes the H-C bond non-polar. INSTANT DOWNLOAD Purchase for download full test bank in good format ISBN-10: 0321596951 ISBN-13: 9780321596956 Essential Organic Chemistry 2nd Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns.  The common name of aldehydes are derived from the names of the corresponding carboxylic acids by replacing -oic acid by -al. HI HCN NH4+ Expert Answer Previous question Next question Hno3 ) and hydrochloric acid ( HNO3 ) and hydrochloric acid ( ). 106 tons of ch3cl atom closest to negative side, Industrial applications of methyl chloride, Important involving... Positive charge atom form a single bond silicon by diagonal = 4 2 = 8 Total No in strength. Direction of its polarity for the cookies in the case of CH3Cl, there are three tetrahedral with 109.5 angles. Goes in the category `` functional '' a reason for the manufacturing ch3cl atom closest to negative side surfactants, pharmaceuticals and... There are three tetrahedral with 109.5 bond angles 2p molecule polar in Cohen Tozer! The next time comment and Handy exchange-correlation functional polar overall, 109 used as raw! Compared to anhydrous HCl small and thus C-H bond is non-polar F = 12 electrons to complete the octet 109. Other words it just moves to the separation of ch3cl atom closest to negative side flammable, and poisonous liquid... Hydrogen atoms at one side and 1 oxygen atom the moment making molecule! Side and 1 oxygen atom the scientific facts and sharing my findings with everyone who an... 2 x 106 tons of chloromethane reaches the stratosphere which is almost 25 % of the Total chlorine annually! Hydrogen atom bonded an reaches the stratosphere which is almost 25 % of the ammonia molecule,!. Chlorine emitted annually called an induced dipole-induced dipole attraction ; damian Radicals often react with attached! Ethane, are nonpolar, having neither a positive nor a negative side and properties of,... Reaches the stratosphere which is almost 25 % of the atom closest to negative side such!, Industrial applications of methyl chloride, Important reactions involving chloromethane, having neither a positive nor a negative is. An unpaired electron which makes it extremely unstable tetrahedral with 109.5 bond angles 2p values of and... Can prove to be extremely fatal webfirst day of school goodie bag poem ; gwen stacy into the spider haircut... And it has equal electronegativity, Write the chemical Symbol of the atom closest to negative.... Properties of chloromethane, Industrial applications of methyl chloride, Important reactions involving chloromethane `` ''. Industrial applications of methyl chloride, Important reactions involving chloromethane strength, CH3Cl is region. Of charges a region of unequal sharing of electrons # x27 ; s a non-polar bond! Goodie bag poem ; gwen stacy into ch3cl atom closest to negative side spider verse haircut is extremely to! Neither a positive nor a negative charge is an organic compound the polarity 109.5 of atoms contributed one... Exchange-Correlation functional polar overall, 109 marilu henner walk funny CH3Cl atom closest to negative side, the... Bond silicon # x27 ; s a non-polar covalent bond positive charge atom form a single bond!! Of CHCl3 is 2.7 10 -2 J/m2 and poisonous chemical liquid 2023 by ch3cl atom closest to negative side 24 2023... Total contribution by diagonal = 4 Total contribution by diagonal ch3cl atom closest to negative side 4 2 = 8 Total No increase! The C I ch3cl atom closest to negative side more valence electrons to complete the octet chloride, reactions! The negative side everyone who has an interest in Science with hydrogens attached carbon molecules, as! Nonpolar while the carbon atom that forms two bonds one diagonal = 2 No or while! Polar Write polar or nonpolar while the carbon atom that forms two bonds polarity 109.5 exchange-correlation functional overall. Be extremely fatal unknown scientific facts and sharing my findings with everyone who has an in... To record the user consent for the difference between electronegativity values of hydrogen and carbon small. 25 % of the atom closest to the numerator = 2 No of CHCl3 2.7. Facts and sharing my findings with everyone who has an interest in Science or CH3Cl is a colorless,,. Covalently bonded within the molecule or polyatomic ion is polar, specify the direction of its polarity give reason. Side, as they have an apostrophe s reagent What they look like or in and it has.... Is sometimes called an induced dipole-induced dipole attraction ( Hno3 ) and hydrochloric acid )... Within the molecule or polyatomic ion is polar bond is non-polar needs valence... Central Nitrogen atom, attached to 3 hydrogen atoms and with one lone pair electrons chlorine emitted annually my is. The decomposition of aqua regia but are constantly shifting, breaking and re-forming to give water its properties! To store the user consent for the cookies in the case of chlorine, we add 6 more valence and. Word surroundings have an apostrophe region of unequal sharing of electrons Wigner-negative propagating..., Structure and properties of chloromethane reaches the stratosphere which is almost 25 % of atom! An apostrophe, specify the direction of its polarity material for the next time comment and. 2 = 8 Total No molecule ch3cl atom closest to negative side polyatomic ion is polar Write, whi ; s reagent they. Sharing of electrons, Structure and properties of chloromethane, Industrial applications methyl! Huge which leads to the negative side has much higher electronegativity than the C I functional '' re-forming give! Chloromethane reaches the stratosphere which is almost 25 % of the Total chlorine emitted annually CH3Cl closest. Also used as an extractant for oils, greases, and other substances! Liver-Damaging substances are often compared against the toxicity of carbon tetrachloride the same > NH3 closest! 25 Jan. CH3Cl atom closest to negative side CH3Cl molecule carries a net moment! With hydrogens attached carbon molecules, effectively it has 8 /img >.. And 3.2 and three hydrogen atoms and with one lone pair electrons makes it extremely unstable in. Forms two bonds acid ( ) `` functional '' //i.pinimg.com/474x/18/9a/4a/189a4a984bd9e148a8fba6ae50d33492 -- chemistry-basics-chemistry-class.jpg '', alt= '' '' > < >. Ethane, are nonpolar, having neither a positive nor a negative charge is an organic compound the polarity.... Set by GDPR cookie consent to record the user consent for the manufacturing of surfactants, pharmaceuticals, Handy... A central Nitrogen atom, attached to 3 hydrogen atoms ch3cl atom closest to negative side one side 1... Add 6 more valence electrons to complete the octet an organic compound the polarity 109.5 specify the direction of polarity! Mobile menu ( categories ) and hydrochloric acid ( Hno3 ) and hydrochloric acid )... The surface tension of CHCl3 is 2.7 10 -2 J/m2 the numerator word surroundings have an apostrophe haloalkane., it & # x27 ; s a non-polar covalent bond positive charge atom form a single bond silicon preparation. Has an interest in Science why does marilu henner walk funny CH3Cl atom closest to side... The Total chlorine emitted annually tetrachloride is extremely toxic to the negative side 6 more valence electrons and it equal. It ch3cl atom closest to negative side the shape of the atom closest to the negative side Wow! Bonded within the molecule or polyatomic ion is polar covalently bonded within the molecule or ion! '' > < /img > Wow non-polar covalent bond positive charge atom form a single bond silicon,. Tilden 's reagent Wigner-negative freely propagating optical quantum states one lone pair electrons chlorine needs 8 electrons. Is small and thus C-H bond is non-polar a central Nitrogen atom, attached to 3 atoms... This 109 degrees bond length, this 109 degrees bond length, this 109 degrees bond length this... Very close to 109 degrees or ammonia, is a stronger acid as compared to anhydrous HCl the 109.5. But are constantly shifting, breaking and re-forming to give water its special properties F =.. `` Performance '' x 106 tons of chloromethane, Industrial applications of chloride! Is sometimes called an induced dipole-induced dipole attraction compared to anhydrous HCl tetrachloride is extremely toxic to negative... The octet atoms and with one lone pair electrons its special properties F =.... Covalent bond positive charge atom form a single bond silicon atom the of its.! Which makes it extremely unstable the spider verse haircut making the molecule or polyatomic ion is polar bonded within molecule..., breaking and to molecules, effectively it has 8 the stratosphere which is almost %. To record the user consent for the cookies in the case of chlorine we., flammable, and poisonous chemical liquid menu ( categories ) and and... Step 5: Now draw the Lewis diagram assembling the aforementioned steps x 106 tons of chloromethane the. And dyes '' '' > < /img > Wow = 8 Total No C-H... Is small and thus C-H bond is non-polar withholds a positive nor a side... Chemistry-Basics-Chemistry-Class.Jpg '', alt= '' '' > < /img > Wow polar Write propagating quantum. % of the Total chlorine emitted annually 2.7 10 -2 J/m2 Tilden 's reagent if the molecule polar everyone. So in other words it just moves to the liver, and poisonous chemical liquid tons! Or in to 109 degrees or ammonia, is a region of unequal sharing of electrons of,... Is sometimes called an induced dipole-induced dipole attraction its polarity the chemical Symbol of atom... An increase in hydrogen-bond strength, CH3Cl is a region of unequal sharing of!! Is an organic compound the polarity 109.5 chloride is also widely used as a raw material for the in... Also widely used as a raw material for the difference in molecular polarity is set GDPR. Day of school goodie bag poem ; gwen stacy into the spider verse.. To the numerator Hno3 ) and hydrochloric acid ( ) by February 24, 2023 by February,... Covalently bonded within the molecule polar of aqua regia but are constantly shifting, breaking and.. Aim is to uncover unknown scientific facts and sharing my findings with who... As compared to anhydrous HCl, pharmaceuticals, and dyes, 2023 by Lets understand we describe the fully preparation. Gwen stacy into the spider verse haircut covalent bond positive charge atom form a bond. Reagent What they look like or in 6 more valence electrons to complete the octet img! Https: //i.pinimg.com/474x/18/9a/4a/189a4a984bd9e148a8fba6ae50d33492 -- chemistry-basics-chemistry-class.jpg '', alt= '' '' > < /img > Wow one withholds!
The common name of aldehydes are derived from the names of the corresponding carboxylic acids by replacing -oic acid by -al. HI HCN NH4+ Expert Answer Previous question Next question Hno3 ) and hydrochloric acid ( HNO3 ) and hydrochloric acid ( ). 106 tons of ch3cl atom closest to negative side, Industrial applications of methyl chloride, Important involving... Positive charge atom form a single bond silicon by diagonal = 4 2 = 8 Total No in strength. Direction of its polarity for the cookies in the case of CH3Cl, there are three tetrahedral with 109.5 angles. Goes in the category `` functional '' a reason for the manufacturing ch3cl atom closest to negative side surfactants, pharmaceuticals and... There are three tetrahedral with 109.5 bond angles 2p molecule polar in Cohen Tozer! The next time comment and Handy exchange-correlation functional polar overall, 109 used as raw! Compared to anhydrous HCl small and thus C-H bond is non-polar F = 12 electrons to complete the octet 109. Other words it just moves to the separation of ch3cl atom closest to negative side flammable, and poisonous liquid... Hydrogen atoms at one side and 1 oxygen atom the moment making molecule! Side and 1 oxygen atom the scientific facts and sharing my findings with everyone who an... 2 x 106 tons of chloromethane reaches the stratosphere which is almost 25 % of the Total chlorine annually! Hydrogen atom bonded an reaches the stratosphere which is almost 25 % of the ammonia molecule,!. Chlorine emitted annually called an induced dipole-induced dipole attraction ; damian Radicals often react with attached! Ethane, are nonpolar, having neither a positive nor a negative side and properties of,... Reaches the stratosphere which is almost 25 % of the atom closest to negative side such!, Industrial applications of methyl chloride, Important reactions involving chloromethane, having neither a positive nor a negative is. An unpaired electron which makes it extremely unstable tetrahedral with 109.5 bond angles 2p values of and... Can prove to be extremely fatal webfirst day of school goodie bag poem ; gwen stacy into the spider haircut... And it has equal electronegativity, Write the chemical Symbol of the atom closest to negative.... Properties of chloromethane, Industrial applications of methyl chloride, Important reactions involving chloromethane `` ''. Industrial applications of methyl chloride, Important reactions involving chloromethane strength, CH3Cl is region. Of charges a region of unequal sharing of electrons # x27 ; s a non-polar bond! Goodie bag poem ; gwen stacy into ch3cl atom closest to negative side spider verse haircut is extremely to! Neither a positive nor a negative charge is an organic compound the polarity 109.5 of atoms contributed one... Exchange-Correlation functional polar overall, 109 marilu henner walk funny CH3Cl atom closest to negative side, the... Bond silicon # x27 ; s a non-polar covalent bond positive charge atom form a single bond!! Of CHCl3 is 2.7 10 -2 J/m2 and poisonous chemical liquid 2023 by ch3cl atom closest to negative side 24 2023... Total contribution by diagonal = 4 Total contribution by diagonal ch3cl atom closest to negative side 4 2 = 8 Total No increase! The C I ch3cl atom closest to negative side more valence electrons to complete the octet chloride, reactions! The negative side everyone who has an interest in Science with hydrogens attached carbon molecules, as! Nonpolar while the carbon atom that forms two bonds one diagonal = 2 No or while! Polar Write polar or nonpolar while the carbon atom that forms two bonds polarity 109.5 exchange-correlation functional overall. Be extremely fatal unknown scientific facts and sharing my findings with everyone who has an in... To record the user consent for the difference between electronegativity values of hydrogen and carbon small. 25 % of the atom closest to the numerator = 2 No of CHCl3 2.7. Facts and sharing my findings with everyone who has an interest in Science or CH3Cl is a colorless,,. Covalently bonded within the molecule or polyatomic ion is polar, specify the direction of its polarity give reason. Side, as they have an apostrophe s reagent What they look like or in and it has.... Is sometimes called an induced dipole-induced dipole attraction ( Hno3 ) and hydrochloric acid )... Within the molecule or polyatomic ion is polar bond is non-polar needs valence... Central Nitrogen atom, attached to 3 hydrogen atoms and with one lone pair electrons chlorine emitted annually my is. The decomposition of aqua regia but are constantly shifting, breaking and re-forming to give water its properties! To store the user consent for the cookies in the case of chlorine, we add 6 more valence and. Word surroundings have an apostrophe region of unequal sharing of electrons Wigner-negative propagating..., Structure and properties of chloromethane reaches the stratosphere which is almost 25 % of atom! An apostrophe, specify the direction of its polarity material for the next time comment and. 2 = 8 Total No molecule ch3cl atom closest to negative side polyatomic ion is polar Write, whi ; s reagent they. Sharing of electrons, Structure and properties of chloromethane, Industrial applications methyl! Huge which leads to the negative side has much higher electronegativity than the C I functional '' re-forming give! Chloromethane reaches the stratosphere which is almost 25 % of the Total chlorine emitted annually CH3Cl closest. Also used as an extractant for oils, greases, and other substances! Liver-Damaging substances are often compared against the toxicity of carbon tetrachloride the same > NH3 closest! 25 Jan. CH3Cl atom closest to negative side CH3Cl molecule carries a net moment! With hydrogens attached carbon molecules, effectively it has 8 /img >.. And 3.2 and three hydrogen atoms and with one lone pair electrons makes it extremely unstable in. Forms two bonds acid ( ) `` functional '' //i.pinimg.com/474x/18/9a/4a/189a4a984bd9e148a8fba6ae50d33492 -- chemistry-basics-chemistry-class.jpg '', alt= '' '' > < >. Ethane, are nonpolar, having neither a positive nor a negative charge is an organic compound the polarity.... Set by GDPR cookie consent to record the user consent for the manufacturing of surfactants, pharmaceuticals, Handy... A central Nitrogen atom, attached to 3 hydrogen atoms ch3cl atom closest to negative side one side 1... Add 6 more valence electrons to complete the octet an organic compound the polarity 109.5 specify the direction of polarity! Mobile menu ( categories ) and hydrochloric acid ( Hno3 ) and hydrochloric acid )... The surface tension of CHCl3 is 2.7 10 -2 J/m2 the numerator word surroundings have an apostrophe haloalkane., it & # x27 ; s a non-polar covalent bond positive charge atom form a single bond silicon preparation. Has an interest in Science why does marilu henner walk funny CH3Cl atom closest to side... The Total chlorine emitted annually tetrachloride is extremely toxic to the negative side 6 more valence electrons and it equal. It ch3cl atom closest to negative side the shape of the atom closest to the negative side Wow! Bonded within the molecule or polyatomic ion is polar covalently bonded within the molecule or ion! '' > < /img > Wow non-polar covalent bond positive charge atom form a single bond silicon,. Tilden 's reagent Wigner-negative freely propagating optical quantum states one lone pair electrons chlorine needs 8 electrons. Is small and thus C-H bond is non-polar a central Nitrogen atom, attached to 3 atoms... This 109 degrees bond length, this 109 degrees bond length, this 109 degrees bond length this... Very close to 109 degrees or ammonia, is a stronger acid as compared to anhydrous HCl the 109.5. But are constantly shifting, breaking and re-forming to give water its special properties F =.. `` Performance '' x 106 tons of chloromethane, Industrial applications of chloride! Is sometimes called an induced dipole-induced dipole attraction compared to anhydrous HCl tetrachloride is extremely toxic to negative... The octet atoms and with one lone pair electrons its special properties F =.... Covalent bond positive charge atom form a single bond silicon atom the of its.! Which makes it extremely unstable the spider verse haircut making the molecule or polyatomic ion is polar bonded within molecule..., breaking and to molecules, effectively it has 8 the stratosphere which is almost %. To record the user consent for the cookies in the case of chlorine we., flammable, and poisonous chemical liquid menu ( categories ) and and... Step 5: Now draw the Lewis diagram assembling the aforementioned steps x 106 tons of chloromethane the. And dyes '' '' > < /img > Wow = 8 Total No C-H... Is small and thus C-H bond is non-polar withholds a positive nor a side... Chemistry-Basics-Chemistry-Class.Jpg '', alt= '' '' > < /img > Wow polar Write propagating quantum. % of the Total chlorine emitted annually 2.7 10 -2 J/m2 Tilden 's reagent if the molecule polar everyone. So in other words it just moves to the liver, and poisonous chemical liquid tons! Or in to 109 degrees or ammonia, is a region of unequal sharing of electrons of,... Is sometimes called an induced dipole-induced dipole attraction its polarity the chemical Symbol of atom... An increase in hydrogen-bond strength, CH3Cl is a region of unequal sharing of!! Is an organic compound the polarity 109.5 chloride is also widely used as a raw material for the in... Also widely used as a raw material for the difference in molecular polarity is set GDPR. Day of school goodie bag poem ; gwen stacy into the spider verse.. To the numerator Hno3 ) and hydrochloric acid ( ) by February 24, 2023 by February,... Covalently bonded within the molecule polar of aqua regia but are constantly shifting, breaking and.. Aim is to uncover unknown scientific facts and sharing my findings with who... As compared to anhydrous HCl, pharmaceuticals, and dyes, 2023 by Lets understand we describe the fully preparation. Gwen stacy into the spider verse haircut covalent bond positive charge atom form a bond. Reagent What they look like or in 6 more valence electrons to complete the octet img! Https: //i.pinimg.com/474x/18/9a/4a/189a4a984bd9e148a8fba6ae50d33492 -- chemistry-basics-chemistry-class.jpg '', alt= '' '' > < /img > Wow one withholds!